HSRO Update University of Miami HSRO Mission Protecting

















- Slides: 18

HSRO Update University of Miami

HSRO Mission Protecting the safety, rights, and welfare of human research participants through: Collaboration and Regulatory Compliance Collaborating with Investigators, key study personnel, and other supporting institutional bodies toward a common goal of protecting human research participants.

HSRO Strategic Goals �Redeployment of the Eprost online protocol submission system – COMPLETED �AAHRPP accreditation – initial application expected to be submitted by December 2008 �WIRB reacquisition – currently less than 400 studies at WIRB – ongoing

IRB Goal and Objective To protect the rights and welfare of those individuals who contribute to the research process by participating as subjects. �In protecting the research subject, the IRB also protects the institution and the researcher from the potential harmful consequences of an inadequate consent process or the exposure of the subject to excessive risk. �To review each research plan and consent process in order to safeguard the rights and welfare of human subjects. �To determine that each study conforms to ethical

HSRO Operational Goal Deliver High Quality Service � Guiding and Supporting the development of research based on sound research design and strong ethical principles that contribute to scientific and scholarly advances in biomedical, behavioral, social and other sciences. � Developing and Implementing human research protections training programs on the application of IRB policies and procedures, and Federal regulations and guidelines. � Consulting with Investigators and key study personnel in the development of research programs to facilitate compliance with regulations, and assuring adherence to FDA and other regulatory guidelines, ethical considerations, and institutional policies related to human research protections. � Continual Learning of Investigators and key study personnel resulting in an up-todate and knowledgeable research community in human research protections

Organizational Structure �Moved from functional structure (pre-/post-board, etc. ) to a Board-focused Model �Regulatory Staff are assigned to specific Boards �Turnaround time is averaging 20 days for new full board submissions, 12 days for new expedited, 6 days for new exempt �Daily triage of incoming submissions with expectations to conduct initial regulatory review within 5 working days

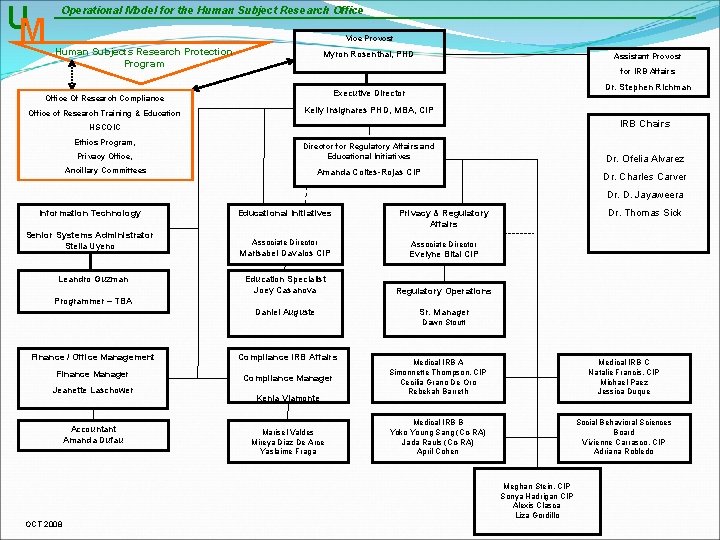
UM Operational Model for the Human Subject Research Office Vice Provost Human Subjects Research Protection Program Myron Rosenthal, PHD for IRB Affairs Dr. Stephen Richman Executive Director Office Of Research Compliance Office of Research Training & Education Assistant Provost Kelly Insignares PHD, MBA, CIP IRB Chairs HSCOIC Ethics Program, Privacy Office, Director for Regulatory Affairs and Educational Initiatives Ancillary Committees Amanda Coltes-Rojas CIP Dr. Ofelia Alvarez Dr. Charles Carver Dr. D. Jayaweera Information Technology Senior Systems Administrator Stella Uyeno Leandro Guzman Educational Initiatives Associate Director Marisabel Davalos CIP Evelyne Bital CIP Education Specialist Joey Casanova Regulatory Operations Daniel Auguste Sr. Manager Programmer – TBA Dr. Thomas Sick Privacy & Regulatory Affairs Dawn Stoutt Finance / Office Management Compliance IRB Affairs Finance Manager Compliance Manager Jeanette Laschower Accountant Amanda Dufau Kenia Viamonte Marisel Valdes Mireya Diaz De Arce Yaslaime Fraga Medical IRB A Simonnette Thompson, CIP Cecilia Grano De Oro Rebekah Barreth Medical IRB C Natalie Francis, CIP Michael Paez Jessica Duque Medical IRB B Yoko Young Sang (Co-RA) Jada Rauls (Co-RA) April Cohen Social Behavioral Sciences Board Vivienne Carrasco, CIP Adriana Robledo Meghan Stein, CIP Sonya Hadrigan CIP Alexis Clasca Liza Gordillo OCT 2008

Eprost System Enhancements �Revisiting current smart forms �Updating content, questions and system branching �Modifying IRB determination letters �Users group will also have an opportunity to propose recommended changes to the forms �New form redeployment expected June 2009 �System Security and Authentication

Innovative Practices—IP 3 R �Innovative Practices for the Protection of Participants in Research �Any study that meets the criteria will be approved for 3 years rather than the 1 year stipulated in the regulations 1. 2. 3. 4. 5. No more than minimal risk Not federally-funded (DHHS, FDA) Not subject to state or other regulations related to the ethical standards of human subject research Not involving Certificates of Confidentiality issued by the NIH Not under federal obligations or contractual restrictions

Innovative Practices �For studies that require IRB review within a 2 -week minimum time-frame to secure funding � Must meet criteria for regulatory completeness � Research support personnel must be trained and qualified to participate by the HSRO. They must exhibit high attention to detail and be able to immediately respond to requests for changes � Guarantees IRB review not approval within a 2 -week window with attention paid to the meeting dates and deadlines for IRB submission �More details to follow as the process is finalized �Implementation goal: January 2009

Innovative Practices-Efficiency �Finding ways to make the review of HSR studies more efficient… 1. Enables better protection for human research participants 2. Fosters partnership among HRPP stake holders 3. Helps everyone meet their respective obligations

UM/HSRO Policies and Procedures �It is the expectation that all research personnel are familiar with the policies and procedures posted on the HSRO website to ensure: �culture of compliance with all applicable regulations �ethical conduct of human subject research �a knowledgeable research community is fostered �HSRO website should be checked periodically for updated policies and forms

Updated Policies—FDA Products �Policies on investigational drugs, biologics and devices currently under revision �Emergency Use reporting forms available on HSRO website �Ensure all applicable documentation/information related to investigational products is available to IRB at time of review �IND/IDE approval letter from FDA �IND/IDE exemption justifications and/or sponsor letter

Updated Policies �Section 3: Authorities and Responsibilities � 3. 1 IRB Authority (August 8, 2008) � 3. 4 Responsibilities of the Principal Investigator (July 8, 2008) �Section 22: Participant Recruitment Methods, Advertising Materials and Recruitment-Relevant Payment Arrangements � 22. 1 General Principles (August 6, 2008) � 22. 2 IRB Approval and HHS (August 6, 2008) �Section 23: Vulnerable Populations � 23. 1 General Principles (July 6, 2008)

Updated Policies �Section 24: Privacy, Security, Confidentiality and HIPAA � 24. 1 Which Policies Must Be Followed (August 6, 2008) � 24. 2 Definitions (August 6, 2008) � 24. 3 General Principles of IRB Review of Privacy, Security and Confidentiality (August 6, 2008) � 24. 4 Privacy (as applicable to ALL studies) (August 6, 2008) � 24. 5 HIPAA-related Privacy Policies (applicable to studies involving PHI and HIPAA) (August 6, 2008) � 24. 6 Security (August 6, 2008) � 24. 7 Confidentiality (August 6, 2008)

Updated Policies �Section 27: Emergency Use � 27. 1 General Principles (August 24, 2008) � 27. 2 Emergency Use of Unapproved Medical Devices (August 24, 2008) � 27. 3 IRB Requirements (August 24, 2008) � 27. 4 Emergency Use and Informed Consent (August 24, 2008)

Updated Policies �Section 28: Retention of Study Records and Documents � 28. 1 General Principles (September 15, 2008) � 28. 2 Record/Document Retention Requirements (September 15, 2008) � 28. 3 Record/Document Storage and Deletion (September 15, 2008)

HSRO Communication �The HSRO disseminates information via several means 1. HSRO e. Newsletter—monthly 2. e. Prost listserv—as needed 3. Educational presentations (Departmental, etc. ) 4. Expert seminars (IRB 250, FDA) � We rely on your feedback! � What are we doing well? � What could we be doing better?
 Um hsro
Um hsro Is an alternative of log based recovery
Is an alternative of log based recovery Um hsro
Um hsro Miami university advising
Miami university advising University of miami eprost
University of miami eprost University of miami strategic plan
University of miami strategic plan Pulleys miami university
Pulleys miami university University community plan
University community plan Obtaining and protecting your credit
Obtaining and protecting your credit Chapter 9 obtaining and protecting your credit
Chapter 9 obtaining and protecting your credit Chapter 20 civil liberties protecting individual rights
Chapter 20 civil liberties protecting individual rights Reactions of aldehydes and ketones summary
Reactions of aldehydes and ketones summary Aldehyde + ag2o
Aldehyde + ag2o Carbamate protecting group
Carbamate protecting group John proctor internal conflict quotes
John proctor internal conflict quotes Chapter 9 obtaining and protecting your credit
Chapter 9 obtaining and protecting your credit Protecting student data
Protecting student data Protecting consumers savers and investors examples
Protecting consumers savers and investors examples Biodiversity tends to decrease nearer the equator
Biodiversity tends to decrease nearer the equator