How Electrons are arranged around the nucleus How




- Slides: 12

How Electrons are arranged around the nucleus

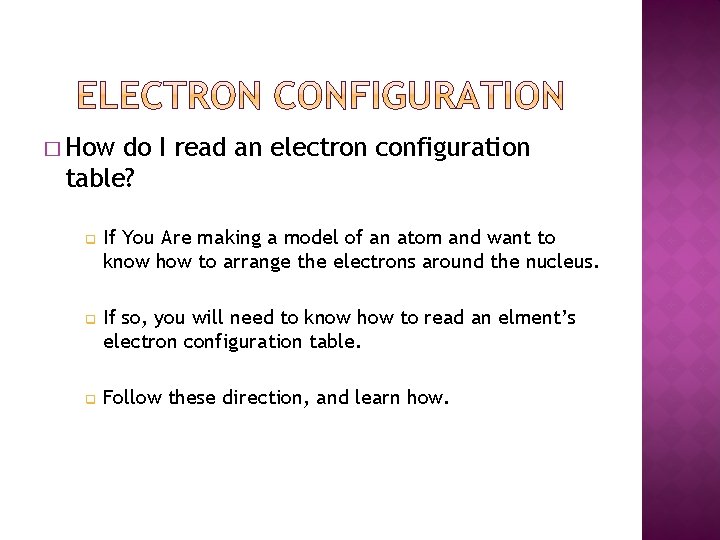
� How do I read an electron configuration table? q q q If You Are making a model of an atom and want to know how to arrange the electrons around the nucleus. If so, you will need to know how to read an elment’s electron configuration table. Follow these direction, and learn how.

� What is an electron configuration table? An electron configuration table is a type of code that describes how many electrons are in each energy level. How the electrons are arranged within each energy level.

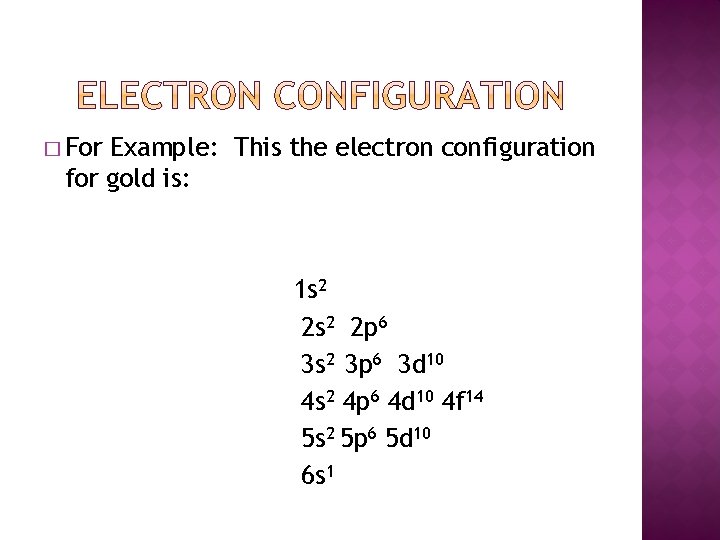
� For Example: This the electron configuration for gold is: 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 4 f 14 5 s 2 5 p 6 5 d 10 6 s 1

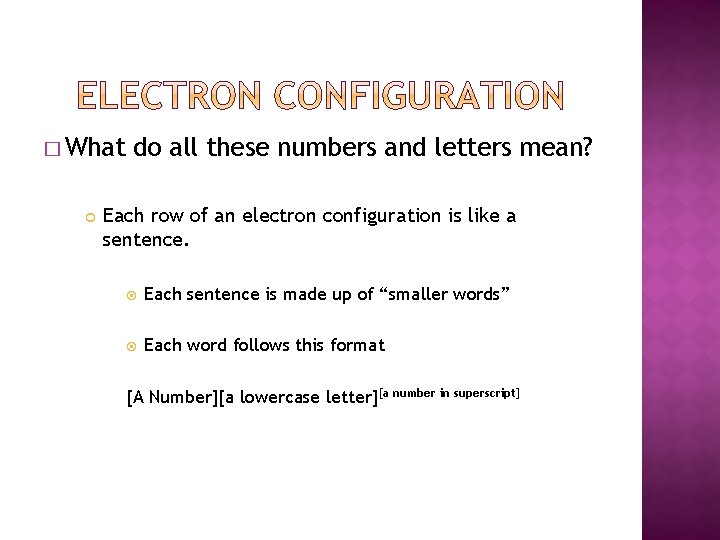
� What do all these numbers and letters mean? Each row of an electron configuration is like a sentence. Each sentence is made up of “smaller words” Each word follows this format [A Number][a lowercase letter][a number in superscript]

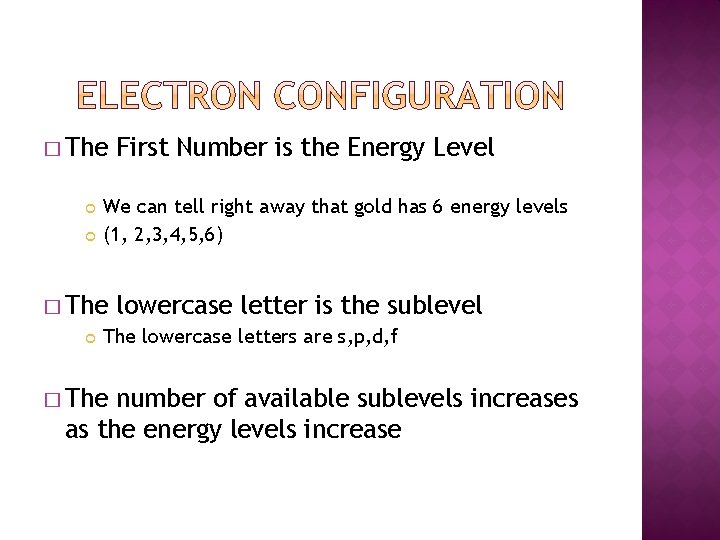
� The We can tell right away that gold has 6 energy levels (1, 2, 3, 4, 5, 6) � The First Number is the Energy Level lowercase letter is the sublevel The lowercase letters are s, p, d, f � The number of available sublevels increases as the energy levels increase

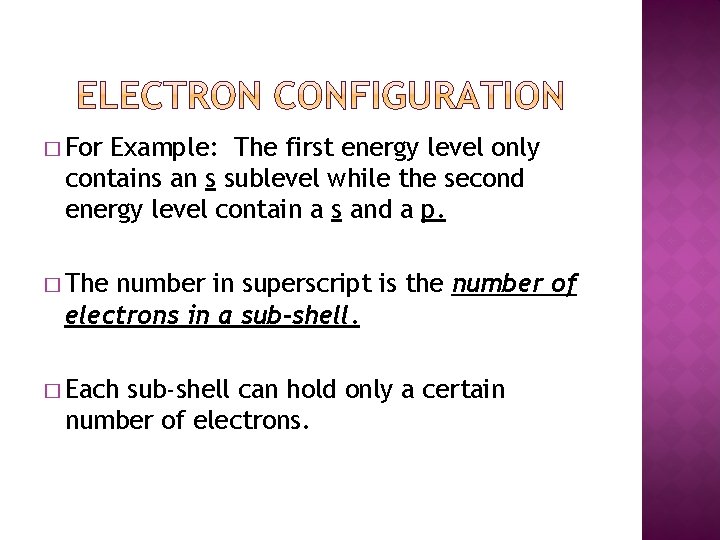
� For Example: The first energy level only contains an s sublevel while the second energy level contain a s and a p. � The number in superscript is the number of electrons in a sub-shell. � Each sub-shell can hold only a certain number of electrons.

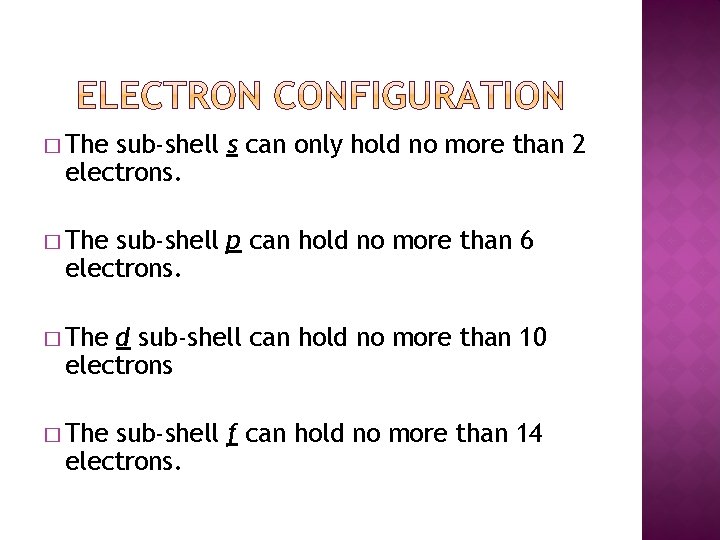
� The sub-shell s can only hold no more than 2 electrons. � The sub-shell p can hold no more than 6 electrons. � The d sub-shell can hold no more than 10 electrons � The sub-shell f can hold no more than 14 electrons.

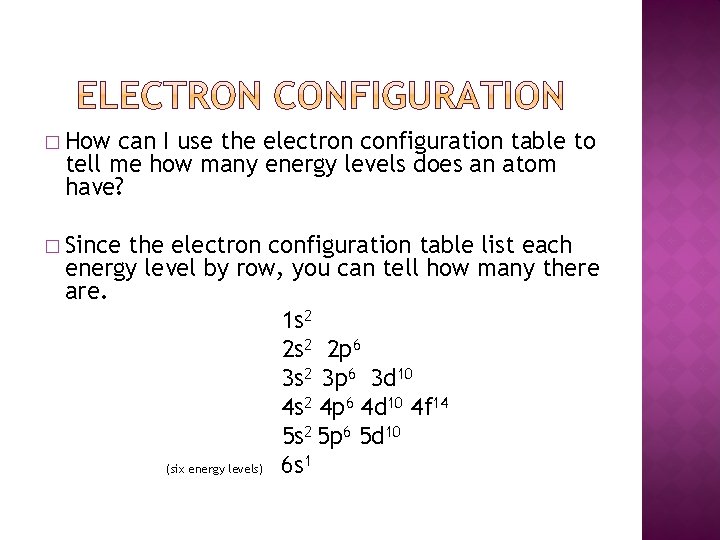
� How can I use the electron configuration table to tell me how many energy levels does an atom have? � Since the electron configuration table list each energy level by row, you can tell how many there are. 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 4 f 14 5 s 2 5 p 6 5 d 10 1 (six energy levels) 6 s

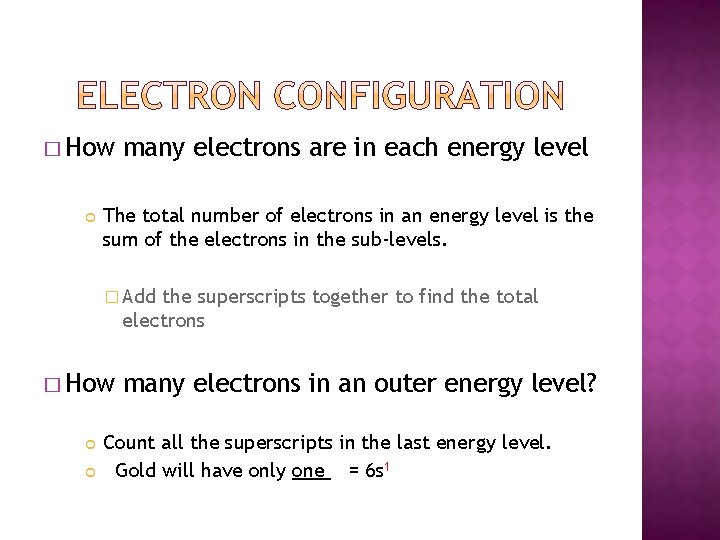
� How many electrons are in each energy level The total number of electrons in an energy level is the sum of the electrons in the sub-levels. � Add the superscripts together to find the total electrons � How many electrons in an outer energy level? Count all the superscripts in the last energy level. Gold will have only one = 6 s 1

� The electrons found in the last energy level is called Valence Electrons. � Example: � Electron Nitrogen Configuration would be: 1 s 2 2 p 3 � 5 valence electrons
