Highlighting Community Priorities for HIV Prevention Trials Jeremiah











- Slides: 13

Highlighting Community Priorities for HIV Prevention Trials Jeremiah Johnson HIV Project Director Treatment Action Group

About Treatment Action Group (TAG) is an independent, activist and community-based research and policy think tank fighting for better treatment, prevention, a vaccine, and a cure for HIV, tuberculosis, and hepatitis C virus. www. treatmentactiongroup. org

Acknowledgments �Report co-authors Richard Jefferys (TAG) and Tim Horn (NASTAD) �Tian Johnson/community advocates behind the Imbizo report �Matt Rose (NMAC) �Mitchell Warren, Stacey Hannah, and the AVAC crew

A Time of Opportunity, A Time of Challenge �Effective primary and secondary prevention! More active pipeline! �A trickier ethical tightrope: balancing obligations to develop new technologies with obligations to provide participants with existing technologies �Enthusiasm from community members for research, scaling up of existing/imminent tools, investment in future choices (incl. microbicides) �Perceived conflict of interest/variability at site level/increased need for investment in community engagement �Opportunity to review research and improve through innovative trial designs �New approaches=increased need for education, engagement challenges

Novel Trial Design Questions/Concerns �Refining statistical approaches/use of correlates to estimate efficacy: Fairly easy to comprehend the benefits. What are the risks? Obviously we do not want efficacy to be overestimated/underestimated Complex: how will community advocates be impartially educated and consulted? �Novel enrollment methods/limits on use of other tools: How will we avoid any perception of discouraging uptake of existing modalities? How do we ensure that we are not interrupting successful Pr. EP usage? Difficult to ethically randomize those in an observational cohort who discontinue existing tools �Levels/Quality of provision of existing tools

Lessons from discussions on Pr. EP Provision �RECOMMENDATIONS** Participants in all biomedical prevention trials should be provided with (quality, enthusiastic) education and referrals for all proven prevention technologies and accompanying services as part of a standard prevention package. Trials should routinely provide data on the number of participants who have been successfully linked to different prevention tools as a way of monitoring impartial education and referrals (Don’t forget U=U) 2. Novel oral Pr. EP regimens must be shown to be non-inferior to oral TDF/FTC and never compared with placebo 1. **From TAG’s white paper The Implications of TDF/FTC Pre-exposure Prophylaxis for Biomedical Prevention Trials. Based on a literature review and community survey.

Lessons from discussions on Pr. EP Provision For well-proven prevention modalities, it should be the standard that researchers opting for only passive referrals or placing restrictions on use of other tools among trial participants must make the case for why they cannot or will not provide or allow these tools in their trial. 3. Farther from ideal provision=shakier ethical ground=increased community engagement Local community and key stakeholder input on provision of comprehensive prevention and novel trial design approaches is essential. CABs should be trained in order to provide meaningful input on novel biomedical trials 5. GPP guidelines should be the standard by which trials operate, and GPP guidelines should be integrated into UNAIDS/WHO and HPTN guidelines. Guidelines must be updated to reflect evolving community perspectives on Pr. EP provision in clinical trials 4.

Additional considerations/Necessary tensions �Key ethical considerations: Modality and medications being tested: is the modality being tested comparable to existing prevention modalities? Additive and synergistic effects? How do partially efficacious tools factor in? Ethical obligation to develop new prevention technologies: is there substantial benefit compared to standard? Approval status of existing prevention tools in trial location and other significant barriers to local access. Do we mirror or exceed real world accessibility? �No “one-size-fits-all” approach. From our survey: Provide free, onsite Pr. EP and related services (48%), referrals to both Pr. EP and services (27. 9%), free TDF/FTC but referrals for services (23. 9%), education only (2. 1%). Several comments on context.

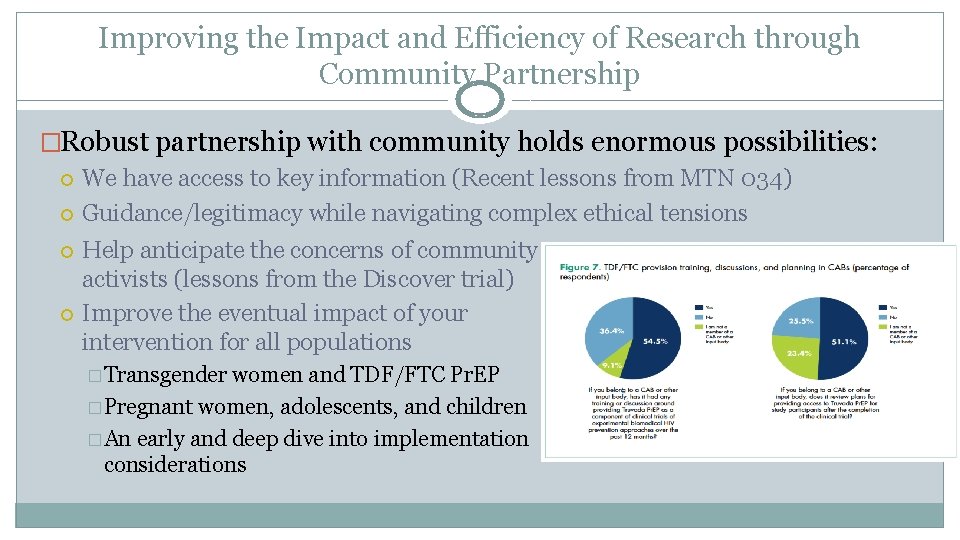
Improving the Impact and Efficiency of Research through Community Partnership �Robust partnership with community holds enormous possibilities: We have access to key information (Recent lessons from MTN 034) Guidance/legitimacy while navigating complex ethical tensions Help anticipate the concerns of community activists (lessons from the Discover trial) Improve the eventual impact of your intervention for all populations � Transgender women and TDF/FTC Pr. EP � Pregnant women, adolescents, and children � An early and deep dive into implementation considerations

Tactics/Principles for Successful Engagement �Mutual Education (researchers, CAB, participants, broader community) �Be clear on how we are defining “Community” �Language, integrity, and power dynamics �More than data extraction: build relationships �Transparency and broad, early, creative engagement. More perspectives=better understanding=more trust

Tactics/Principles for Successful Engagement �Selecting site based on social/economic context + epidemiology �Diversity in research leadership helps with retention and engagement (HPTN 073, Princess Pr. EP) �Using data for efficient recruitment (lessons from HVTN 703), to determine resources required for robust engagement, understand quality of engagement, for accountability and monitoring �Track both benefits and the risks of engagement (HPTN 074) �Early plans for pre, during, and post engagement

It’s not just about “the community” �Advocates exist beyond potential trial participants, trial communities, site-level CABs �Increasingly, HIV-focused advocates are skillfully linking HIV research/trials to broader HIV agendas at country, regional, and global levels �Examples: Vaccine Interest Group, Uganda Africa Free of New HIV Infections South Africa National Stakeholder Engagement Forum (SAMRC, AVAC) Malawi Civil Society Forum Afro. CAB EATG, ECAB – Europe

More Information �Imbizo Report (VARG) https: //issuu. com/thevarg/docs/imbizo_report_2018_email (site will be back up after Nov. 7 th) �TAG’s report on Pr. EP provision http: //www. treatmentactiongroup. org/users/hiv-prep-prevention-trials �Good Participatory Practice: https: //www. avac. org/good-participatorypractice �Who’s Choice is it Anyway? (IRMA) http: //rectalmicrobicides. org/resources/reports-materials/ �HIVR 4 P session: Beyond Placebo (Tuesday Session) http: //webcasts. hivr 4 p. org/yi/2018/23? link=nav&linkc=date
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Stakeholders in hiv prevention
Stakeholders in hiv prevention Global hiv prevention coalition
Global hiv prevention coalition Which type of melanin lends black and brown colors to hair?
Which type of melanin lends black and brown colors to hair? Types of facial makeup
Types of facial makeup Highlighting strategy
Highlighting strategy Syntax highlighting
Syntax highlighting Graphic highlighting in emails
Graphic highlighting in emails Ndsu corn variety trials
Ndsu corn variety trials York trials unit
York trials unit Nida clinical trial network
Nida clinical trial network Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Japanese bridging studies
Japanese bridging studies Ccea
Ccea

























