Herv MARION DVM VICH Secretariat What is VICH






















- Slides: 27

- Hervé MARION, DVM VICH Secretariat What is VICH, History and Objectives

I. III. IV. 2 What is VICH – the History Role & Objectives Conclusion

I. What is VICH ? 3

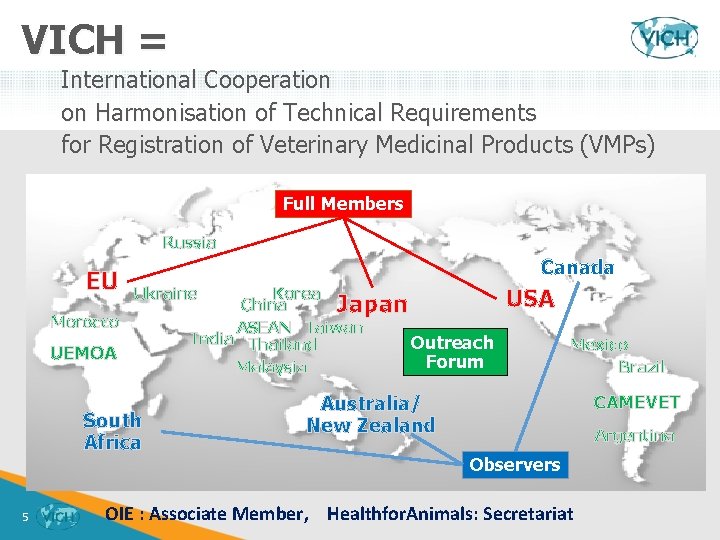
What is VICH? VICH = International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products Ø International cooperation programme US-JAPAN-EU (+ AUS/NZ + Canada + South Africa as observers) Ø Discussion Forum for Regulatory Authorities and Industry Ø 4

VICH = International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VMPs) Full Members Russia EU Canada Ukraine Morocco UEMOA South Africa 5 Korea China Japan ASEAN Taiwan India Thailand Outreach Forum Malaysia USA Mexico Brazil Australia/ New Zealand CAMEVET Argentina Observers OIE : Associate Member, Healthfor. Animals: Secretariat

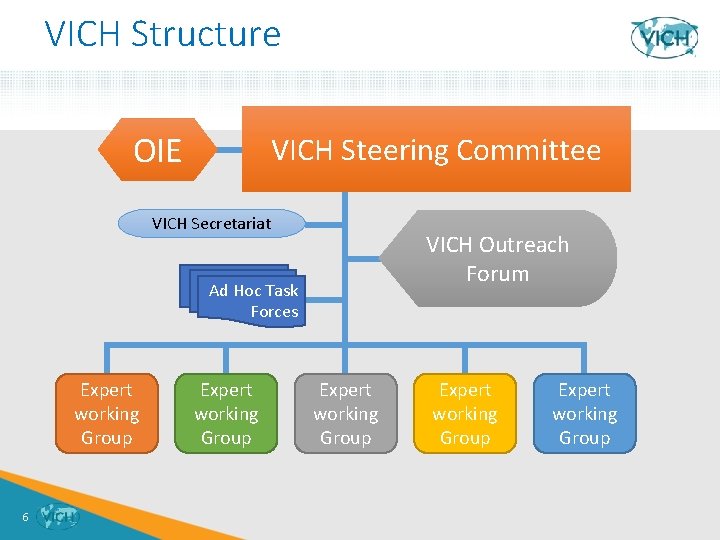
VICH Structure OIE VICH Steering Committee VICH Secretariat VICH Outreach Forum Ad Hoc Task Forces Expert working Group 6 Expert working Group

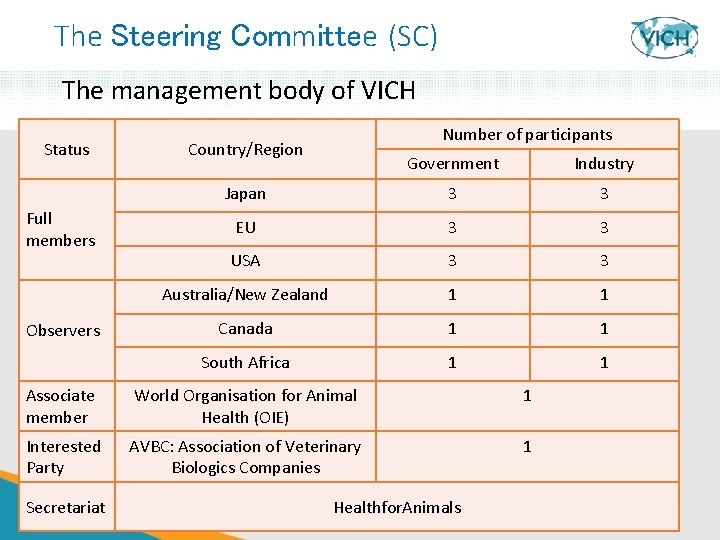
The Steering Committee (SC) The management body of VICH Status Full members Observers Number of participants Country/Region Government Industry Japan 3 3 EU 3 3 USA 3 3 Australia/New Zealand 1 1 Canada 1 1 South Africa 1 1 Associate member World Organisation for Animal Health (OIE) 1 Interested Party AVBC: Association of Veterinary Biologics Companies 1 Secretariat 7 Healthfor. Animals 7

The VICH Steering Committee ü Is the decision making body of VICH and drives the process ü Determines the priority items based on concept papers prepared by its members ü Sets up the appropriate Expert Working Groups (EWGs) topic leaders and EWG chairpersons; and appoints ü Approves the draft Guidelines prepared by EWGs before release for public consultation ü Approves (ONLY the regulatory authorities from the EU, Japan and the USA) the final Guidelines for implementation in the member regions ü Is responsible for a programme of monitoring maintenance and review of Guidelines 8

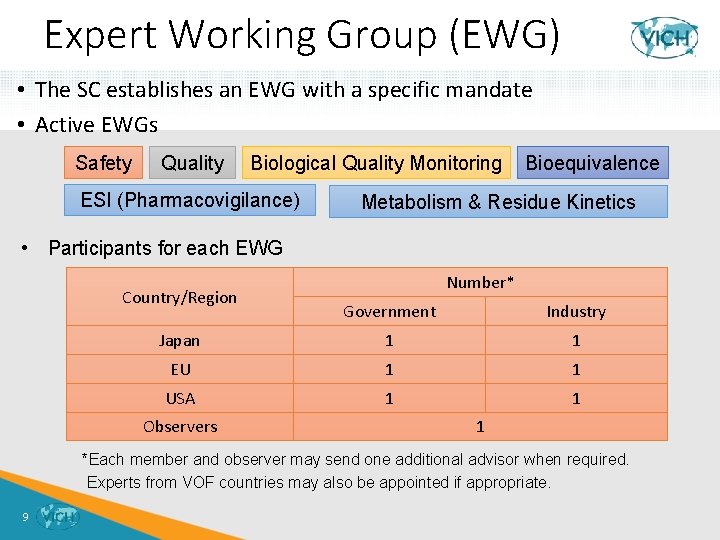
Expert Working Group (EWG) • The SC establishes an EWG with a specific mandate • Active EWGs Safety Quality Biological Quality Monitoring ESI (Pharmacovigilance) Bioequivalence Metabolism & Residue Kinetics • Participants for each EWG Country/Region Number* Government Industry Japan 1 1 EU 1 1 USA 1 1 Observers 1 *Each member and observer may send one additional advisor when required. Experts from VOF countries may also be appointed if appropriate. 9

II. VICH The History 10

VICH History & Milestones 1980’s - 90’s First talks & Meetings on VMP harmonisation 1990 - ICH (human medicines) 1994 - 95 OIE ad hoc Group on the VMP Harmonisation April 1996 1 st VICH Steering Committee in the OIE headquarters in Paris, France Nov. 1999 1 st VICH Public Conference in Brussels, Belgium Oct. 2002 2 nd VICH Public Conference and 11 th Steering Committee meeting in Tokyo, Japan May 2005 3 rd VICH Public Conference and 16 th Steering Committee meeting in Washington DC, USA June 2010 4 th VICH Public Conference , 24 th Steering Committee and plenary exchange on Global Outreach Strategy in the OIE headquarters November 2011 Contact meeting with selected non-VICH country representatives in Tokyo, Japan June 2012 1 st VICH Outreach Forum meeting in Brussels, Belgium October 2015 5 th VICH Public Conference, 6 th VICH Outreach Forum meeting and 32 nd Steering 11 Committee in Tokyo, Japan 11

VICH History International Meetings Rotation between the 3 member regions 32 Steering Committee meetings 6 VICH Outreach Forum meetings 5 VICH Public Conferences Expert Working Groups work through e-mails, teleconferences and face-to-face meetings to progress their work 12 Every 9 months Every 5 years Ad hoc and ongoing

The VICH Outreach Forum (VOF) • Chaired by VICH/OIE for: • Wider international harmonisation • Raising awareness of VICH • Good governance of VMPs worldwide • Matters to be addressed: • • How to participate in VICH work Practical issues for accepting and using VICH Guidelines Sharing of translations of Guidelines Collating comments from the initial stage of Guideline creation, etc…. • Current members Continent Eurasia Africa 13 America Country Regional Organisation China, India, Korea Malaysia, Taiwan, Thailand Russia, Ukraine ASEAN Morocco UEMOA Argentina, Brazil, Mexico CAMEVET

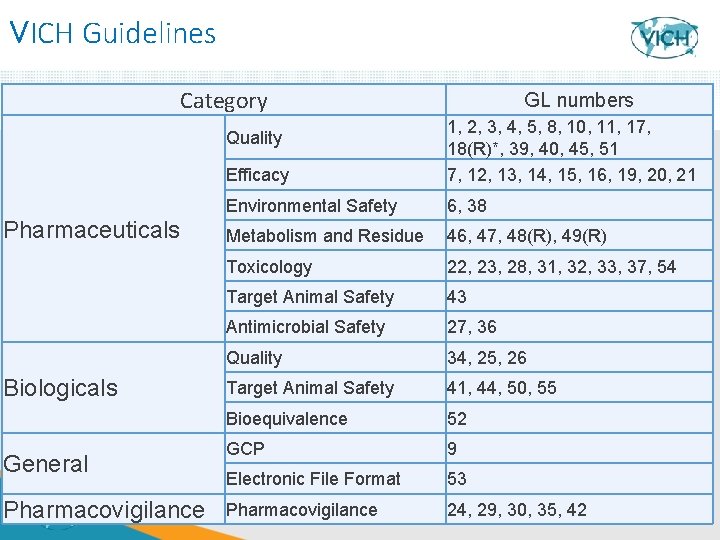
VICH Guidelines Category Efficacy 1, 2, 3, 4, 5, 8, 10, 11, 17, 18(R)*, 39, 40, 45, 51 7, 12, 13, 14, 15, 16, 19, 20, 21 Environmental Safety 6, 38 Metabolism and Residue 46, 47, 48(R), 49(R) Toxicology 22, 23, 28, 31, 32, 33, 37, 54 Target Animal Safety 43 Antimicrobial Safety 27, 36 Quality 34, 25, 26 Target Animal Safety 41, 44, 50, 55 Bioequivalence 52 GCP 9 Electronic File Format 53 Pharmacovigilance 24, 29, 30, 1435, 42 Quality Pharmaceuticals Biologicals General Pharmacovigilance 14 GL numbers

III. VICH Role and Objectives 15

What is the role of VICH? • To harmonise technical requirements for data necessary for registration of a veterinary medicinal product • To develop and implement VICH Guidelines ü Study and testing methodology • Quality, safety and efficacy (including bioequivalence) ü Post-marketing safety monitoring • 16 Pharmacovigilance

It is NOT the role of VICH to: • Provide guidance to establish regulatory systems and regulations for marketing authorisations for Veterinary Medicinal Products • Decide which studies are necessary to obtain a marketing authorisation • Assess data or provide guidance on the assessment approach • Grant marketing authorisations • Establish safety standards These are typically the roles of national competent authorities and governments! 17

VICH Objectives ü Establish and implement harmonised requirements for veterinary medicines in the VICH regions, which • Meet high standards of Quality, Safety & Efficacy to protect public health, animal health & welfare and the environment • Minimise the use of test animals and costs of product development ü Provide a basis for wider international harmonisation of technical requirements ü Ensure efficient processes for maintaining and monitoring consistent interpretation of data requirements following implementation ü Provide technical guidance enabling response to significant emerging global issues and science of relevance 18

VICH Guiding Principles ü The decision making process in VICH should be through consensus ü Procedures should ensure the smooth and consistent functioning of the process for preparation, consultation and adoption of Guidelines ü New topics for development of Guidelines are agreed following evaluation of importance and feasibility of project; requires acceptance of all full VICH members ü Harmonised requirements should replace corresponding regional requirements ü Transparent and cost-effective procedures, open for public comments • Consultation by all regulatory authorities in VICH • Consultation procedure by dissemination to OIE Member Countries through OIE • VICH public website 19

The VICH Process • Thorough selection of topics by the SC based on assessment of benefits and feasibility for harmonisation and resources requirements • Work mandated by the SC to Expert Working Groups i. e. the SC monitors progress of Expert Working Groups and provides support and direction • Elaboration and adoption of Guidelines in a 9 -step procedure • Taking particular note of ICH Guidelines taking account of veterinary specific needs • Consequent need for maintaining and updating existing guidelines on a regular basis 20

Development of a VICH Guideline: The 9 step procedure 21 Step 1 Concept paper to propose issue Review by SC Appointment of Topic Leader/Chairman Step 2 EWG to produce draft Guideline Step 3 SC to approve draft Guideline for consultation Step 4 Public consultation in the regions Step 5 EWG to review comments and finalise Guideline Step 6 SC to adopt final Guideline Step 7 -8 Implementation of Guideline Step 9 Recommendation for review 9 step procedure

Rights and obligations of VICH Members • Members have pledged to implement all finalised VICH Guidelines • Members participate in Steering Committee meetings and in Expert Working Group meetings • Members consult with stakeholders concerning draft and final VICH Guidelines • Members are permitted to sign-off of Guidelines (in final steps regulators only) • Members chair Steering Committee meetings and Expert Working Group meetings 22

IV. Conclusion 23

Achievements Ø Confidence building and close collaboration between the participants since 1996! • Considerable improvements of harmonization of data requirements between regions, thus ü Reduction of animal testing ü Reduction of costs • Better understanding of regulations and concerns in the other regions • Discussion forum between scientific experts from both Regulatory agencies and the Animal Health companies • Contribute to the Global One Health approach 24

Achievements • All decisions in the SC and the EWGs are made by consensus • Unique opportunity for regulators and industry to discuss topics openly enabling a pooling of expertise to jointly draft guidelines on regulatory data requirements • Opportunity to update regional standards • Acceleration of Veterinary Medicinal product development for Livestock & Companion Animals • Increase availability of Veterinary Medicines • Increased Product Safety and Consumer Safety 25

The VICH public website (http: //www. vichsec. org) 26 26

THANK YOU FOR YOUR ATTENTION! 27
 Asean secretariat
Asean secretariat Digital secretariat chhattisgarh
Digital secretariat chhattisgarh Trans kalahari corridor secretariat
Trans kalahari corridor secretariat Honours secretariat
Honours secretariat Public appointments secretariat
Public appointments secretariat General secretariat for development planning
General secretariat for development planning Secretariat scene
Secretariat scene Secretariat family tree
Secretariat family tree Exemple rapport de stage
Exemple rapport de stage Cites secretariat
Cites secretariat Agri benchmark
Agri benchmark Literacy and numeracy secretariat
Literacy and numeracy secretariat Pacific island forum secretariat
Pacific island forum secretariat Honours and appointments secretariat
Honours and appointments secretariat Unep secretariat
Unep secretariat Institute of secretariat training and management
Institute of secretariat training and management Roger clemmons dvm
Roger clemmons dvm Roger clemmons dvm
Roger clemmons dvm Wendy blount
Wendy blount Wei qi booster and stasis breaker
Wei qi booster and stasis breaker Wendy blount dvm
Wendy blount dvm Wendy blount dvm
Wendy blount dvm Wendy blount dvm
Wendy blount dvm Patricia joran nude
Patricia joran nude Canine lateral saphenous vein
Canine lateral saphenous vein Roger clemmons dvm
Roger clemmons dvm Speuter
Speuter Integrating type dvm
Integrating type dvm