Heat treatment Characteristics preparation processes application and advantagesdisadvantages






- Slides: 9

Heat treatment Characteristics, preparation, processes, application and advantages/disadvantages of using the following heat treatments when altering the properties of metals: • Annealing • Hardening and Tempering • Case Hardening

Annealing I • Annealing — is one process by which steel is softened and has its ductility restored. • Process Annealing is used to treat work-hardened parts made out of low-Carbon steels (< 0. 25% Carbon). This allows the parts to be soft enough to undergo further cold working without fracturing. • Stress Relief Annealing is used to reduce residual stresses in large castings, welded parts and coldformed parts.

• Copper, steel, silver, and brass, this process is performed by substantially heating the material (generally until glowing) for a while and allowing it to cool. • Copper, silver and brass can be cooled slowly in air or quickly by quenching in water. • Ferrous metals - must be cooled slowly to anneal. • Softening is done to reduce strength or hardness, remove residual stresses in a piece of metal. • Restoring ductility is a necessary operation when a large amount of cold working is to be performed.

Hardening • When steel is heated above the critical point (until it glows cherry red) and then quenched, cooling is so rapid that the structure of the steel is “frozen”. • The steel is intensely hard and brittle. • This is the first stage in a two stage process known as “quenching & tempering”.

Quenching & Tempering I • Quenching makes the metal hard and brittle. • This is improved by a second heating known as Tempering. • The steel is heated below the critical point (when it shows a blue tempering colour) • This results in a slight decrease in hardness but the steel becomes tougher. • The exact temperature for Tempering depends on the final use of the steel.

Quenching & Tempering II • The degree of hardness depends on the carbon content of the steel. • Carbon content below 0. 3% shows no noticeable change. • Max. hardness is achieved with 0. 7 % carbon content

Case Hardening • Articles made from low carbon steel are given a hard skin by carburising the surfaces, thus enclosing the soft iron core in a case of high carbon steel. • The articles retain the toughness of mild steel whilst the hardened surfaces can withstand wear. • The process is carried out by heating articles in the presence of carbon rich solids, liquids or gases. • The greater the heat and the longer to time the greater the degree of Carbon absorbtion.

Questions… 1. Explain how repeat bending of mild steel wire will result in it breaking in two. 2. What is meant by the term ‘annealing’? 3. How would you anneal aluminium? 4. How would you anneal mild steel? 5. How can high carbon steel be hardened by heat? 6. Why does hardened steel need to be tempered? 7. Illustrate the stages of tempering a screwdriver head. (State the colour that you need to heat the blade to). 8. How is mild steel treated so that it can be hardened? 9. Case hardened mild steel is tough. Why? 10. Between the temperatures of 230°C and 300°C, steel can be said to have a built-in thermometer. How can this be true?

• • • Heat Treatment Theory Demonstrate Brittleness of Steel. Demonstrate the Malleability of Steel. Demonstrate changing the properties of Steel. Demonstrate Forging Demonstrate Shaping Demonstrate Hardening (glows Cherry Red) Demonstrate Quenching (in Oil) Demonstrate Tempering (turns Blue)
 Concurrent processes are processes that
Concurrent processes are processes that For the two processes shown which of the following is true
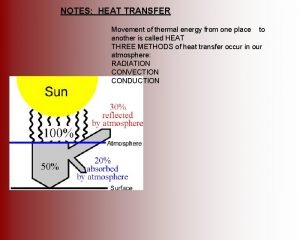
For the two processes shown which of the following is true Identify the following processes of transfer of heat.
Identify the following processes of transfer of heat. Wholesale heat treating
Wholesale heat treating Normalizing adalah
Normalizing adalah Normalizing heat treatment
Normalizing heat treatment Heat treatment cost calculator
Heat treatment cost calculator Heat treatment phase diagram
Heat treatment phase diagram Purpose of heat treatment
Purpose of heat treatment Purpose of heat treatment
Purpose of heat treatment