H 3 in the Early Universe YuShan M

- Slides: 11

H 3 in the Early Universe + Yu-Shan M. Chen and Takeshi Oka Department of Chemistry and Department of Astronomy and Astrophysics The Enrico Fermi Institute, University of Chicago 74 th International Symposium on Molecular Spectroscopy University of Illinois, Urbana-Champaign June 21 st, 2019

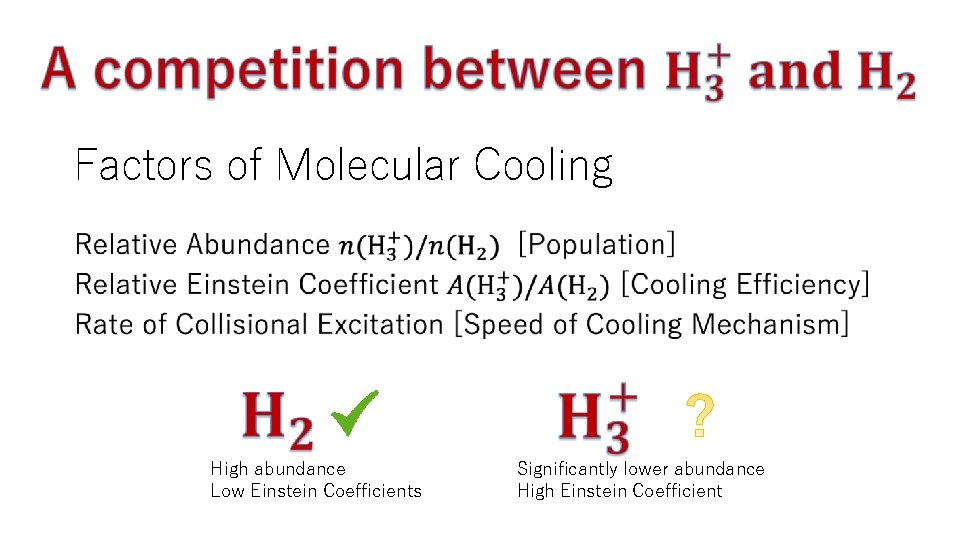
Factors of Molecular Cooling • High abundance Low Einstein Coefficients Significantly lower abundance High Einstein Coefficient

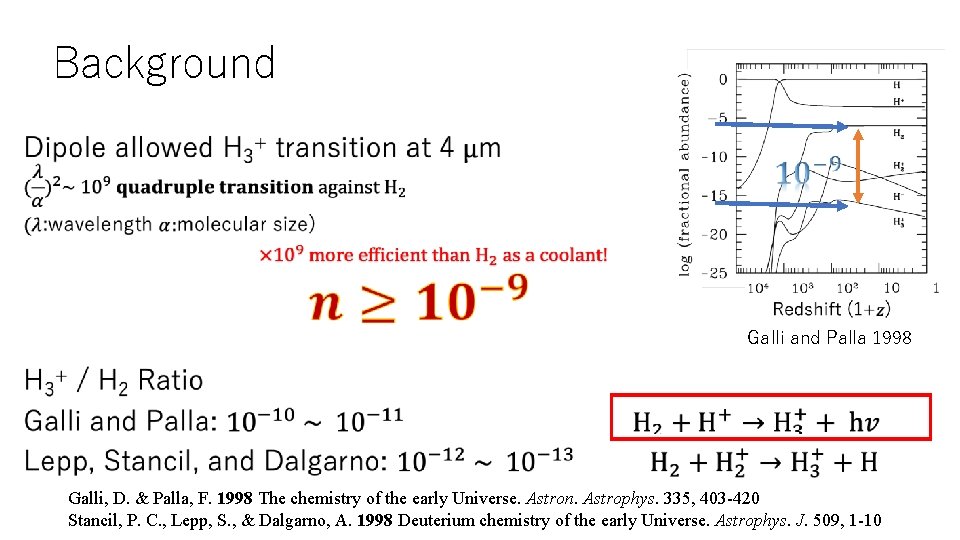
Background • Galli and Palla 1998 Galli, D. & Palla, F. 1998 The chemistry of the early Universe. Astron. Astrophys. 335, 403 -420 Stancil, P. C. , Lepp, S. , & Dalgarno, A. 1998 Deuterium chemistry of the early Universe. Astrophys. J. 509, 1 -10

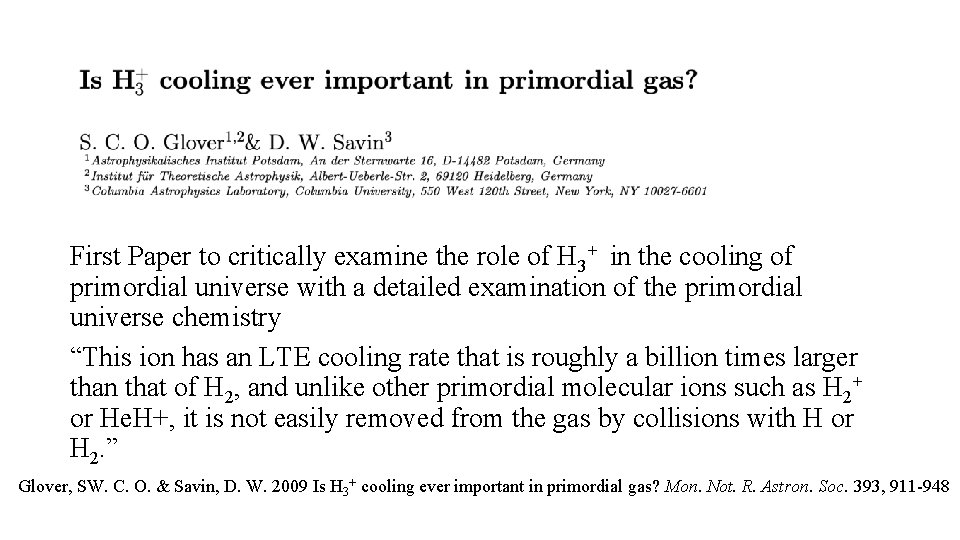
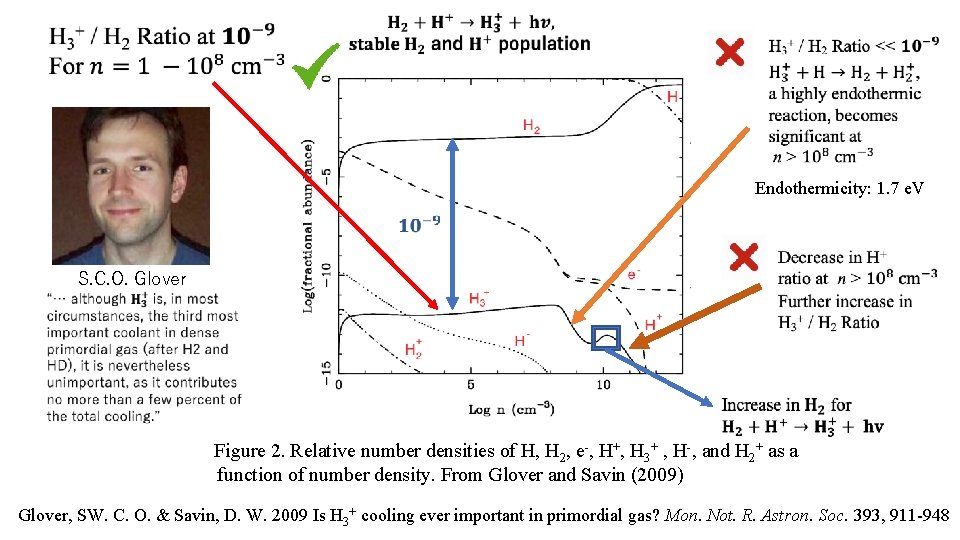
First Paper to critically examine the role of H 3+ in the cooling of primordial universe with a detailed examination of the primordial universe chemistry “This ion has an LTE cooling rate that is roughly a billion times larger than that of H 2, and unlike other primordial molecular ions such as H 2+ or He. H+, it is not easily removed from the gas by collisions with H or H 2. ” Glover, SW. C. O. & Savin, D. W. 2009 Is H 3+ cooling ever important in primordial gas? Mon. Not. R. Astron. Soc. 393, 911 -948

Endothermicity: 1. 7 e. V S. C. O. Glover Figure 2. Relative number densities of H, H 2, e-, H+, H 3+ , H-, and H 2+ as a function of number density. From Glover and Savin (2009) Glover, SW. C. O. & Savin, D. W. 2009 Is H 3+ cooling ever important in primordial gas? Mon. Not. R. Astron. Soc. 393, 911 -948

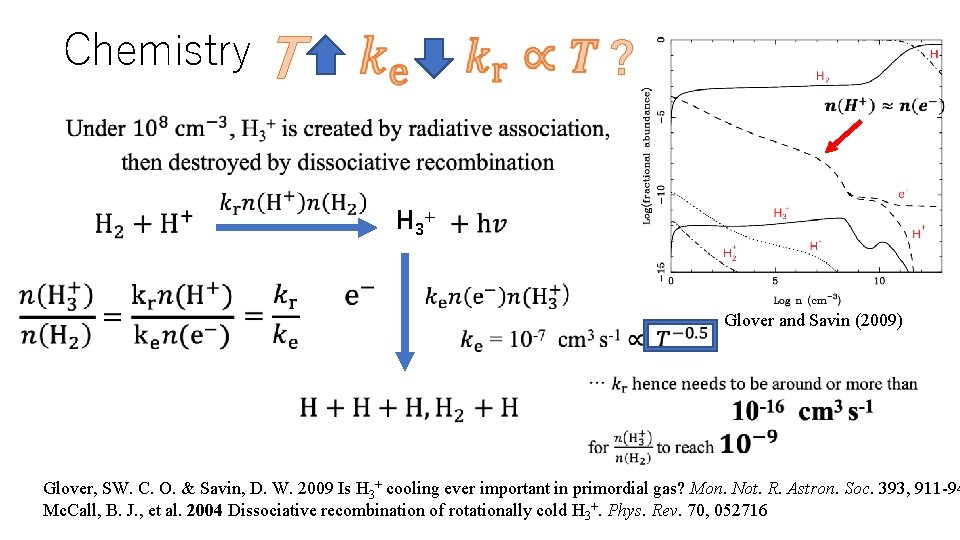
Chemistry T ? H 3+ Glover and Savin (2009) Glover, SW. C. O. & Savin, D. W. 2009 Is H 3+ cooling ever important in primordial gas? Mon. Not. R. Astron. Soc. 393, 911 -94 Mc. Call, B. J. , et al. 2004 Dissociative recombination of rotationally cold H 3+. Phys. Rev. 70, 052716

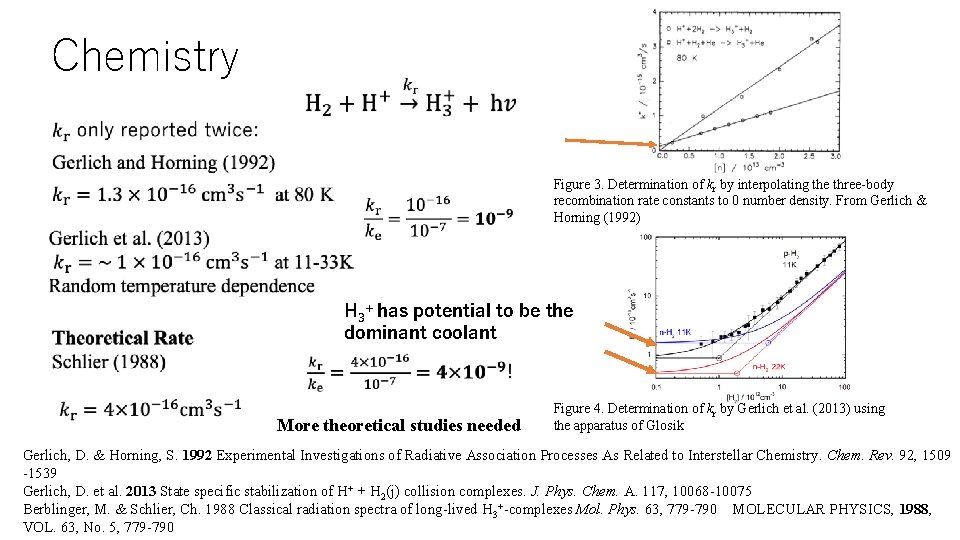
Chemistry • Figure 3. Determination of kr by interpolating the three-body recombination rate constants to 0 number density. From Gerlich & Horning (1992) H 3+ has potential to be the dominant coolant More theoretical studies needed Figure 4. Determination of kr by Gerlich et al. (2013) using the apparatus of Glosik Gerlich, D. & Horning, S. 1992 Experimental Investigations of Radiative Association Processes As Related to Interstellar Chemistry. Chem. Rev. 92, 1509 -1539 Gerlich, D. et al. 2013 State specific stabilization of H+ + H 2(j) collision complexes. J. Phys. Chem. A. 117, 10068 -10075 Berblinger, M. & Schlier, Ch. 1988 Classical radiation spectra of long-lived H 3+-complexes Mol. Phys. 63, 779 -790 MOLECULAR PHYSICS, 1988, VOL. 63, No. 5, 779 -790

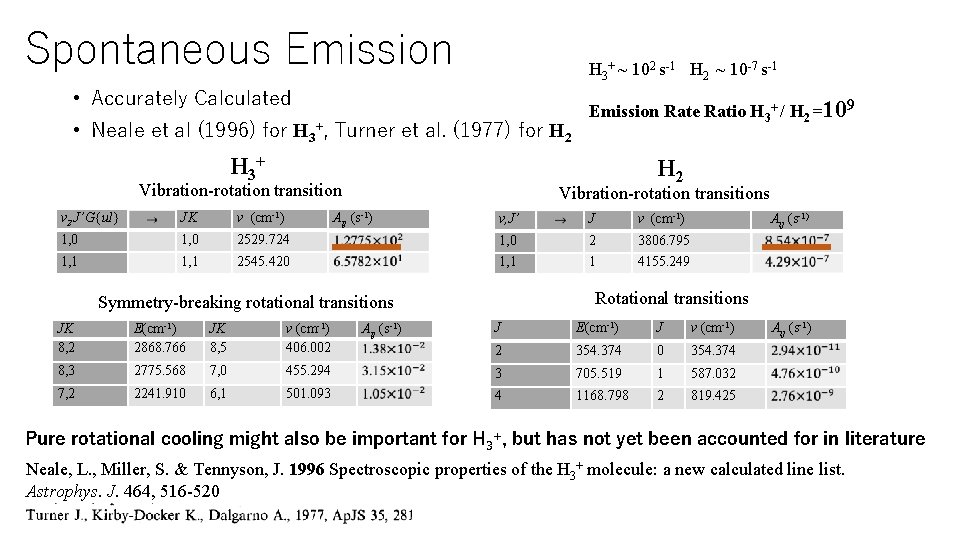
Spontaneous Emission H 3+ ~ 102 s-1 H 2 ~ 10 -7 s-1 • Accurately Calculated • Neale et al (1996) for H 3+, Turner et al. (1977) for H 2 H 3+ H 2 Vibration-rotation transition v 2 J’G{ul} JK ν (cm-1) 1, 0 1, 1 Emission Rate Ratio H 3+ / H 2 =109 Vibration-rotation transitions Aij (s-1) v, J’ J ν (cm-1) 2529. 724 1, 0 2 3806. 795 2545. 420 1, 1 1 4155. 249 Aij (s-1) Rotational transitions Symmetry-breaking rotational transitions J E(cm-1) J v (cm-1) 2 354. 374 0 354. 374 455. 294 3 705. 519 1 587. 032 501. 093 4 1168. 798 2 819. 425 JK 8, 2 E(cm-1) 2868. 766 JK 8, 5 v (cm-1) 406. 002 8, 3 2775. 568 7, 0 7, 2 2241. 910 6, 1 Aij (s-1) Pure rotational cooling might also be important for H 3+, but has not yet been accounted for in literature Neale, L. , Miller, S. & Tennyson, J. 1996 Spectroscopic properties of the H 3+ molecule: a new calculated line list. Astrophys. J. 464, 516 -520

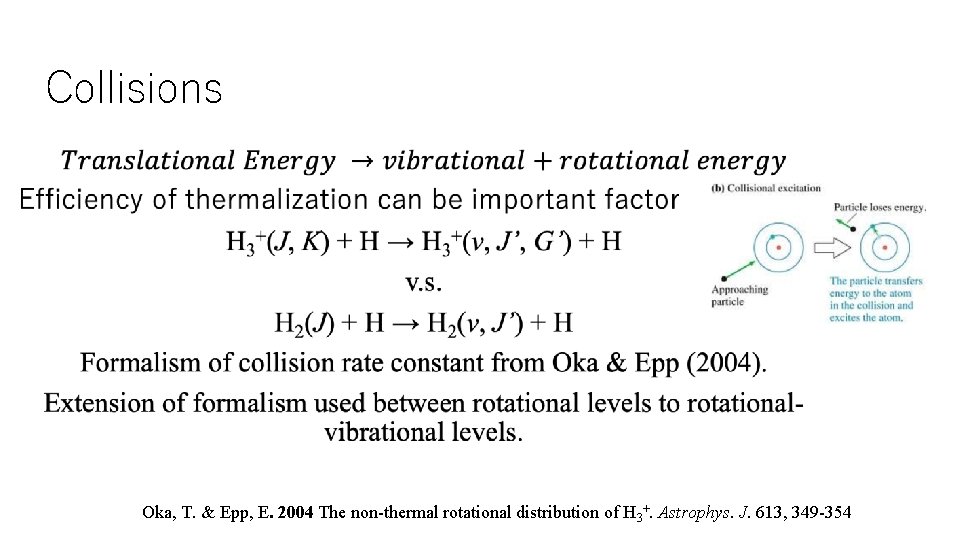
Collisions • Oka, T. & Epp, E. 2004 The non-thermal rotational distribution of H 3+. Astrophys. J. 613, 349 -354

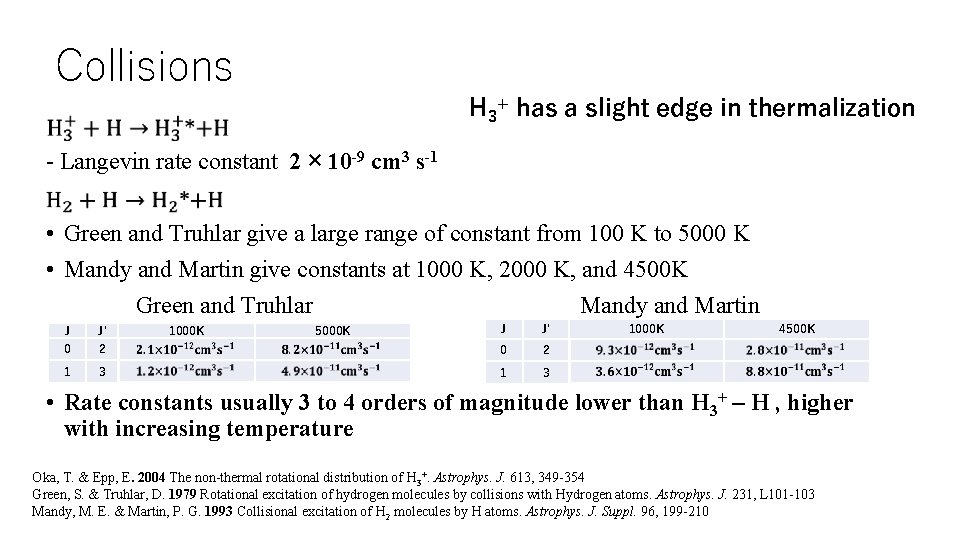
Collisions H 3+ has a slight edge in thermalization - Langevin rate constant 2 × 10 -9 cm 3 s-1 • Green and Truhlar give a large range of constant from 100 K to 5000 K • Mandy and Martin give constants at 1000 K, 2000 K, and 4500 K Green and Truhlar Mandy and Martin J J' 2 0 2 3 1 3 J J' 0 1 1000 K 5000 K 1000 K 4500 K • Rate constants usually 3 to 4 orders of magnitude lower than H 3+ – H , higher with increasing temperature Oka, T. & Epp, E. 2004 The non-thermal rotational distribution of H 3+. Astrophys. J. 613, 349 -354 Green, S. & Truhlar, D. 1979 Rotational excitation of hydrogen molecules by collisions with Hydrogen atoms. Astrophys. J. 231, L 101 -103 Mandy, M. E. & Martin, P. G. 1993 Collisional excitation of H 2 molecules by H atoms. Astrophys. J. Suppl. 96, 199 -210

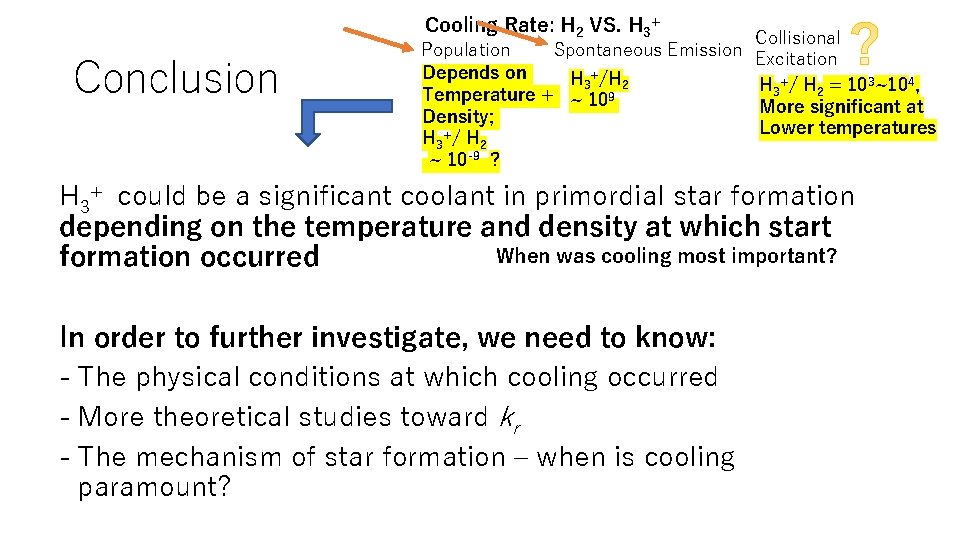
Cooling Rate: H 2 VS. H 3+ Conclusion Population Spontaneous Emission Depends on H 3+/H 2 Temperature + ~ 109 Density; H 3+/ H 2 ~ 10 -9 ? Collisional Excitation H 3+/ H 2 = 103~104, More significant at Lower temperatures H 3+ could be a significant coolant in primordial star formation depending on the temperature and density at which start When was cooling most important? formation occurred In order to further investigate, we need to know: - The physical conditions at which cooling occurred - More theoretical studies toward kr - The mechanism of star formation – when is cooling paramount?