Group 7 The Halogens Group 7 the halogens








- Slides: 16

Group 7 The Halogens

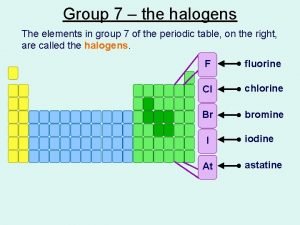
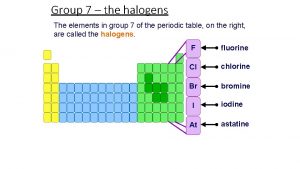
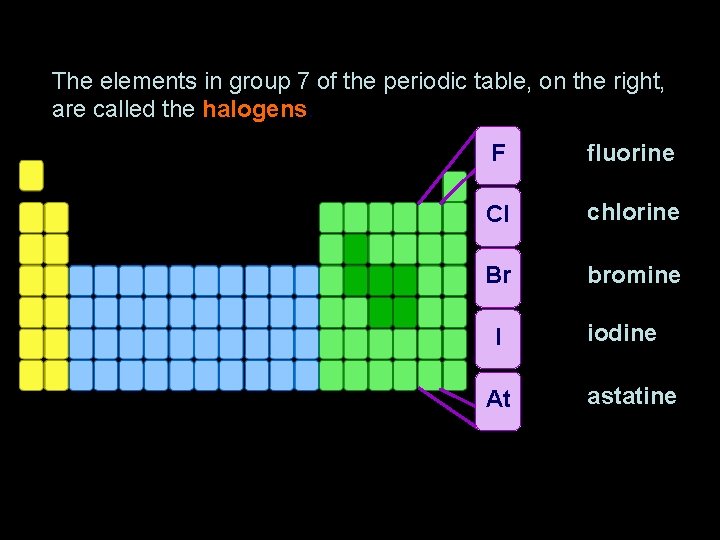
Group 7 – the halogens The elements in group 7 of the periodic table, on the right, are called the halogens. F fluorine Cl chlorine Br bromine I At iodine astatine

Why are they called the ‘halogens’? Halogens are very reactive non metals. They are all toxic or harmful because they are so reactive. Before antiseptics, iodine was used to clean wounds as it is harmful to all things, including bacteria. They are also never found free in nature because of their reactivity – they are found as compounds with metals. These halogen-metal compounds are salts, which give halogens their name – ‘halo-gen’ means ‘salt-former’.

What are the general properties of the halogens? All the halogens are: l Diatomic non-metals and so do not conduct electricity l brittle and crumbly when solid l poisonous and smelly. l Very reactive and strong oxidising agents. They become darker in colour down the group: is pale yellow is yellow-green is red-brown is grey

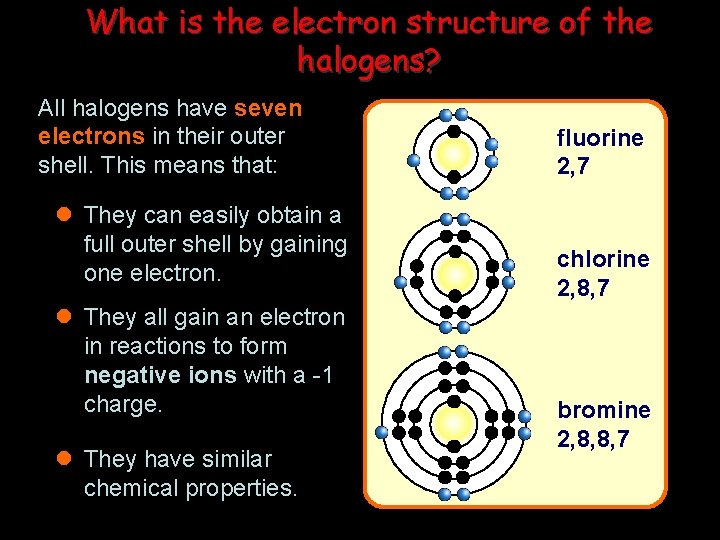
What is the electron structure of the halogens? All halogens have seven electrons in their outer shell. This means that: l They can easily obtain a full outer shell by gaining one electron. l They all gain an electron in reactions to form negative ions with a -1 charge. l They have similar chemical properties. fluorine 2, 7 chlorine 2, 8, 7 bromine 2, 8, 8, 7

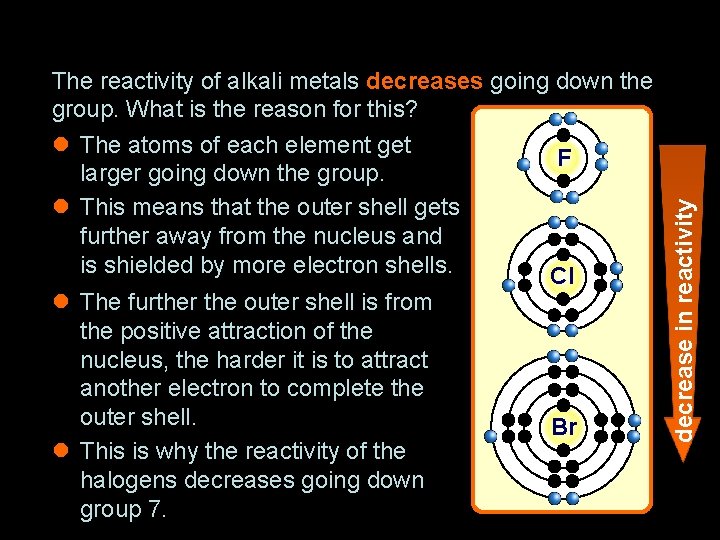
The reactivity of alkali metals decreases going down the group. What is the reason for this? l The atoms of each element get F larger going down the group. l This means that the outer shell gets further away from the nucleus and is shielded by more electron shells. Cl l The further the outer shell is from the positive attraction of the nucleus, the harder it is to attract another electron to complete the outer shell. l This is why the reactivity of the halogens decreases going down group 7. Br decrease in reactivity How does electron structure affect reactivity?

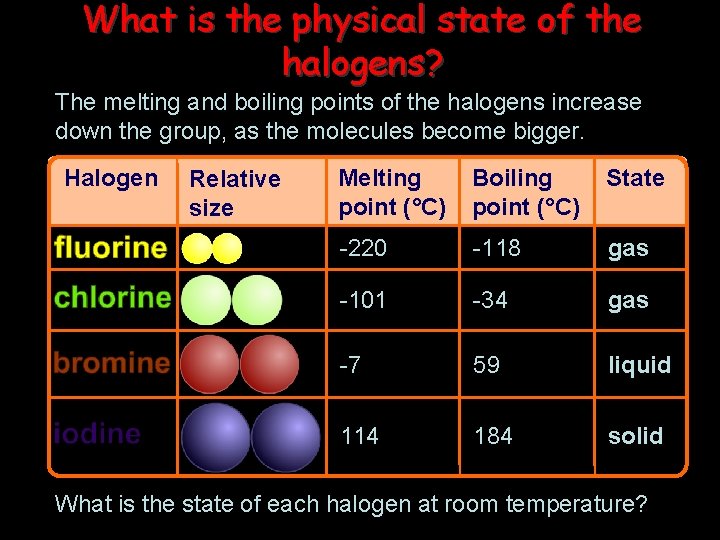
What is the physical state of the halogens? The melting and boiling points of the halogens increase down the group, as the molecules become bigger. Halogen Relative size Melting point (°C) Boiling point (°C) State -220 -118 gas -101 -34 gas -7 59 liquid 114 184 solid What is the state of each halogen at room temperature?

Halogen vapours Bromine and iodine are not gaseous, but have low boiling points. This means that they produce vapour at relatively low temperature. They are volatile. Bromine produces some red-brown vapour, seen here above the liquid bromine in the jar. When iodine is heated gently, it changes directly from a solid to a gas without first becoming a liquid. This is called sublimation.

Halogens – what do they look like? Chlorine Bromine Iodine

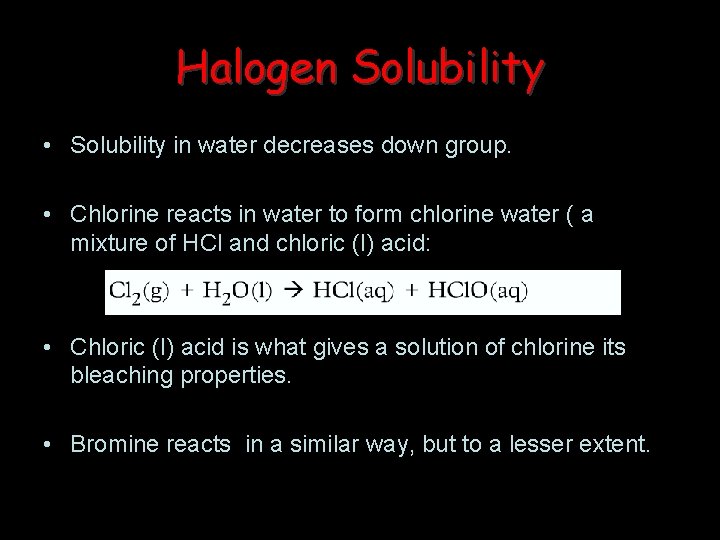
Halogen Solubility • Solubility in water decreases down group. • Chlorine reacts in water to form chlorine water ( a mixture of HCl and chloric (I) acid: • Chloric (I) acid is what gives a solution of chlorine its bleaching properties. • Bromine reacts in a similar way, but to a lesser extent.

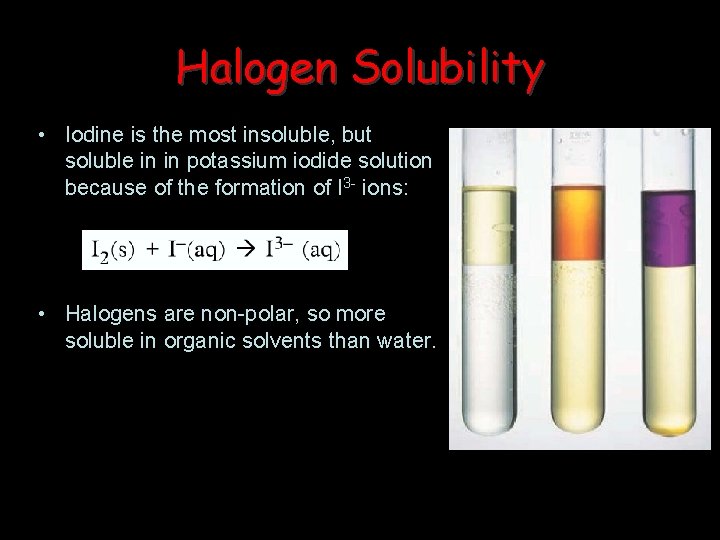
Halogen Solubility • Iodine is the most insoluble, but soluble in in potassium iodide solution because of the formation of I 3 - ions: • Halogens are non-polar, so more soluble in organic solvents than water.

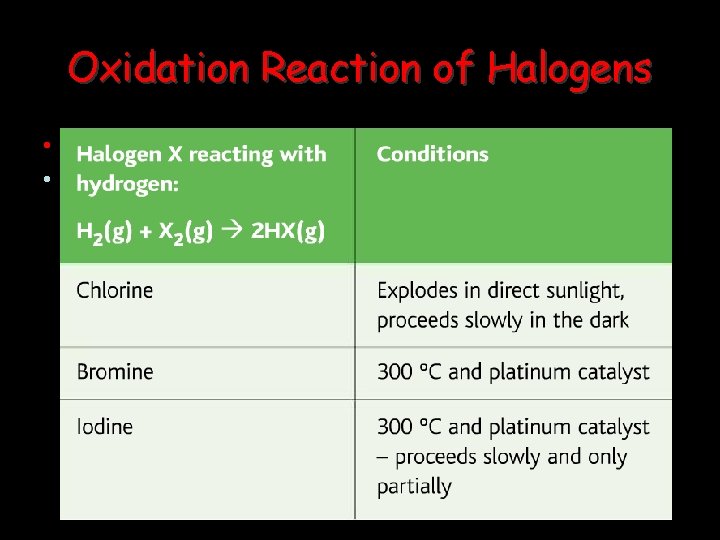
Oxidation Reaction of Halogens • With Metals: • React strongly with electropositive metals, removing the outer electrons to become reduced themselves.

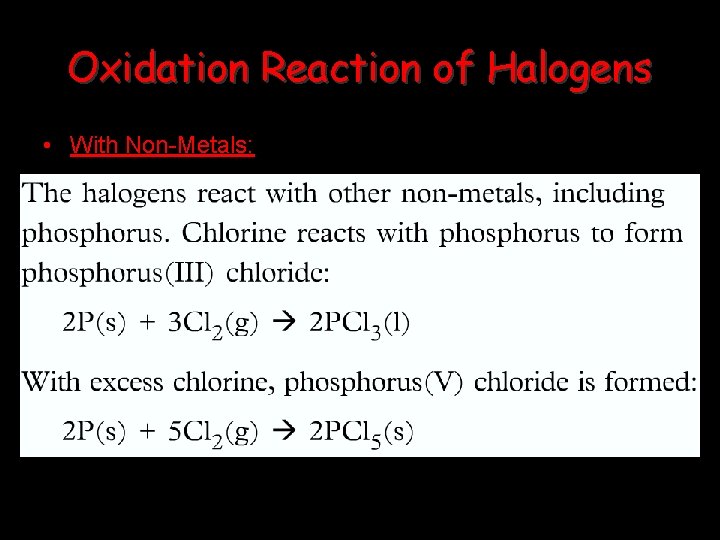
Oxidation Reaction of Halogens • With Non-Metals: • The halogen usually achieves a noble gas configuration by forming a covalent bond:

Oxidation Reaction of Halogens • With Non-Metals:

Oxidation Reaction of Halogens • With Iron(II) chloride solution:

Questions
 Group 7 halogens
Group 7 halogens Chemsheets as 1063 answers group 2
Chemsheets as 1063 answers group 2 Halogens electron structure
Halogens electron structure ჰალოგენები
ჰალოგენები Halogens in periodic table
Halogens in periodic table Atom of halogen
Atom of halogen What are halogens
What are halogens Bromine solubility
Bromine solubility Halogens in periodic table
Halogens in periodic table Halogens examples
Halogens examples Halogens in periodic table
Halogens in periodic table Group with 6 valence electrons
Group with 6 valence electrons The nature of covalent bonding
The nature of covalent bonding Addition of halogens to alkenes
Addition of halogens to alkenes Ionic radius of halogens
Ionic radius of halogens What are halogens
What are halogens Alkaline earth metal family
Alkaline earth metal family