GLYCOSIDES Terpenoids 6 th lecture3 rd stage1 st

















- Slides: 25

GLYCOSIDES& Terpenoids 6 th lecture/3 rd stage/1 st semester

A. DIGITALIS 1. Digitalis purpurea : (Foxglove; Purple foxglove) Digitalis purpurea(Scrophulariaceae) [ The word purpurea has been derived from the purple colour of flowers]. It has been reported that digitalis essentially contains three important primary glycosides namely: Purpurea glycoside A, Purpurea glycoside B, and Purpurea glycoside C, which upon hydrolysis give rise to digitoxin, gitoxin and gitalin respectively. These secondary glcosides on further hydrolysis yields noncarbohydrate moieties (called aglycones or genins) digitoxigenin, gitoxigenin and gitaligenin respectively.


Uses 1. Digitalis enhances the force of contraction of heart muscle and increase cardiac output. Digitalis together with its various marketed preparations are employed profusely as vital cardiotonics in the management and control of different kinds of congestive heart failure, atrial flutter, atrial fibrillation, supraventricular tachycardia and premature extra systoles. 2. Digitalis has a tendency to exert an overall cumulative effect in the body, and hence it gets eliminated rather gradually. Therefore, it is extremely important to monitor the dosage regimen by a physician whether he relies on branded products or natural drug preparations eg. , Digoxin injection and tablets(Lanoxin).

2. Digitalis Lanata: White Foxglove leaf Digitalis lanata(Scrophulariaceae) It contains Lanatoside A, Lanatoside B, and Lanatoside C. Digoxin derived from Lanatoside C _______________________ 3. Digitalis lutea: Straw Foxglove Digitalis lutea(Scrophulariaceae) Uses 1. It is used as a common substitute for the official drug. 2. It is potent as D. purpurea. 3. It is mostly used for the same purpose as that of D. purpurea. __________________________ 4. Digitalis Thapsi: (Spanish Foxglove) Digitalis thapsi(Scrophulariaceae) Uses Its therapeutic efficacy is almost 1. 25 to 3 times more potent than D. purpurea and its actions are similar.

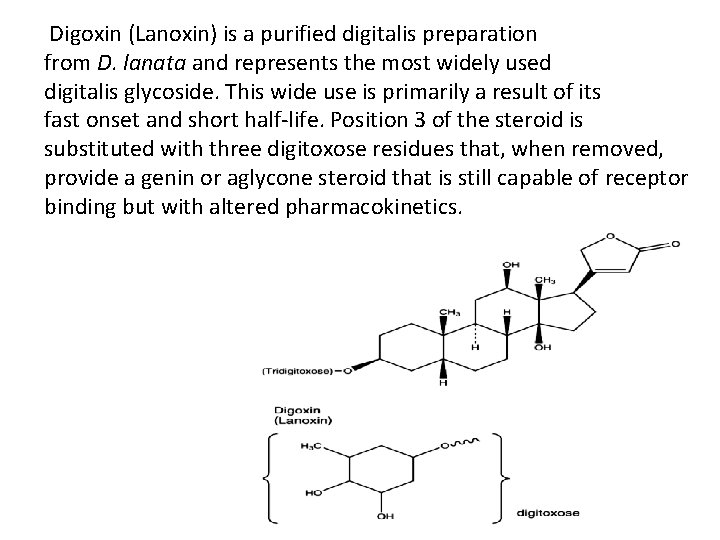
Digoxin (Lanoxin) is a purified digitalis preparation from D. lanata and represents the most widely used digitalis glycoside. This wide use is primarily a result of its fast onset and short half-life. Position 3 of the steroid is substituted with three digitoxose residues that, when removed, provide a genin or aglycone steroid that is still capable of receptor binding but with altered pharmacokinetics.

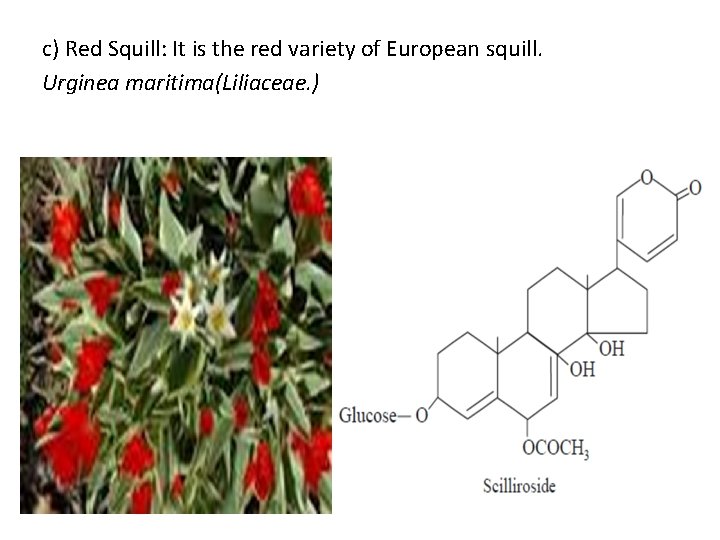
B. Squill It contains two glycosides in their purest form, namely: Scillaren A and Scillaren B. These two naturally occuring glyosides are usually present in the crude drug in the ratio 2: 1 (i. e. , 2 parts of Scillaren A and 1 part of Scillaren B). Generally, the squill is available in three varieties, namely: (a) European Squill (b) Indian Squill, and (c) Red Squill ___________________________ a) European Squill: (Sea onion, White Squill) Scilla maritima(Liliaceae)

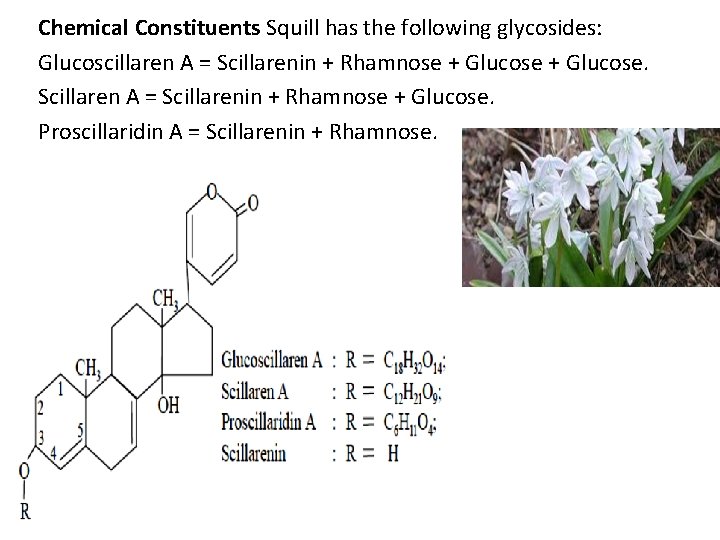
Chemical Constituents Squill has the following glycosides: Glucoscillaren A = Scillarenin + Rhamnose + Glucose. Scillaren A = Scillarenin + Rhamnose + Glucose. Proscillaridin A = Scillarenin + Rhamnose.

Uses 1. It is a potent cardiotonic without any cumulative effect (unlike Digitalis). 2. It is mostly employed in small doses as an effective expectorant specially in chronic bronchitis. 3. It causes emesis in relatively higher doses. 4. The squill glycosides usually produce diuresis. 5. squill glycosides possess high therapeutic index and rapid elimination they invariably maintain compensation in such patients where a prolonged treatment is required.

b) Indian Squill: Scilla Urginea indica(Liliaceae) The two major cardiac glycosides present in the drug are Scillaren A and Scillaren B. Uses 1. It is largely employed as a cardiotonic. 2. It is used as a very effective expectorant both in asthma and chronic bronchitis. 3. It possesses anticancer activity against human carcinoma. Disadvantage: It is in no way a perfect replacement for Digitalis since it possesses not only irritant effect but also is very poorly absorbed systemically.

c) Red Squill: It is the red variety of European squill. Urginea maritima(Liliaceae. )

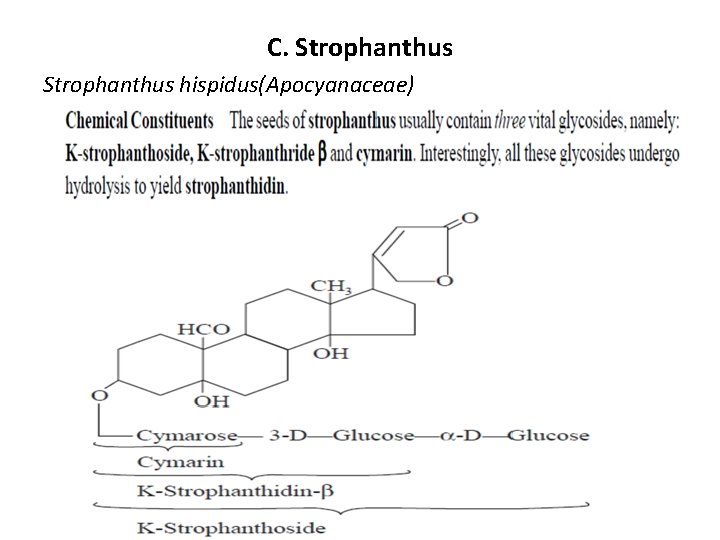
C. Strophanthus hispidus(Apocyanaceae)

In K-Strophanthoside , the aglycone is known as strophanthidin that has the following characteristic features: (a) Three—OH moieties at positions C-3, C-5 and C-14. (b) An aldehydic (—CHO) function is present at C-10 which being an essential requirement. (c) At C-17 an unsaturated 5 -membered lactone ring, and (d) At C-3 an ‘O’ atom forms a bridge to the sugar compotent(s) essentially comprising of cymarose, beta-D-glucose and alpha -D-glucose. Acidic hydrolysis of K-strophanthoside gives rise to the aglycone strophanthidin along with a triose sugar known as strophanthotriose that comprise of one mole of cymarose and two moles of glucose. Uses It is used intravenously for treating emergency cardiac conditions. However, orally strophanthin is not so active.

10 Bitter Glycosides 1. Picrorhiza: (Indian Gentian) Picrorhiza kurroa(Scrophulariaceae) Chemical Constituents: The therapeutically potent constituents of the drug essentially comprises of three vital bitter glycosides, namely: Picroside I, Picroside II and Kutkoside. Uses 1. It is mostly employed as a vital tonic 2. It is also used as a febrifuge. 3. It exerts its action as a laxative. 4. It also finds its usefulness in the treatment of jaundice. 5. Its alcohlic extract exhibits remarkable antibacterial effect.

2. Chirata: Bitter stick Swerlia chirata (Gentianaceae) Chemical Constituents: amarogentin and chiratin. Uses 1. It is invariably used as a bitter tonic. 2. It also finds its use as a febrifuge. 3. It is employed in dyspepsia. 4. It has been recommended as a diuretic and in epilepsy. 5. Industrially, it is extensively used in dyeing cotton cloth.

11 Miscellaneous Glycosides 1. Steroidal Alkaloidal Glycosides: a. Rubijervine Solanum lycopersicum (Solanaceae) (Tomato) Used as antifungal b. Solanine Solanum tuberosum (Solanaceae) (Potato)

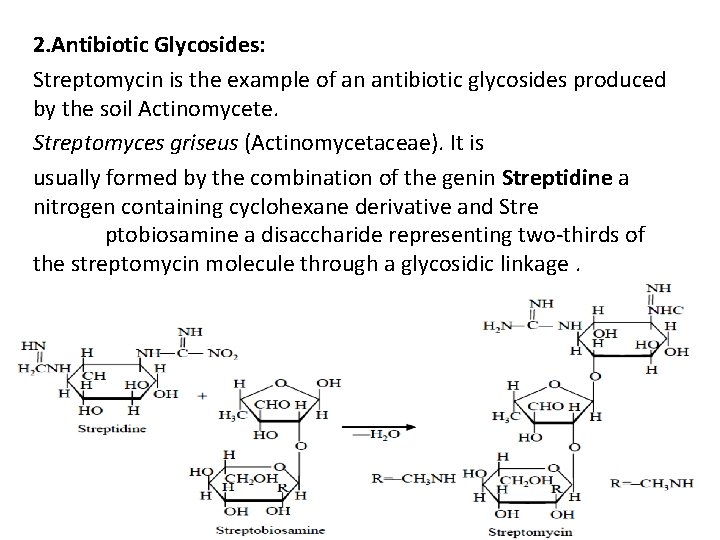
2. Antibiotic Glycosides: Streptomycin is the example of an antibiotic glycosides produced by the soil Actinomycete. Streptomyces griseus (Actinomycetaceae). It is usually formed by the combination of the genin Streptidine a nitrogen containing cyclohexane derivative and Stre ptobiosamine a disaccharide representing two-thirds of the streptomycin molecule through a glycosidic linkage.

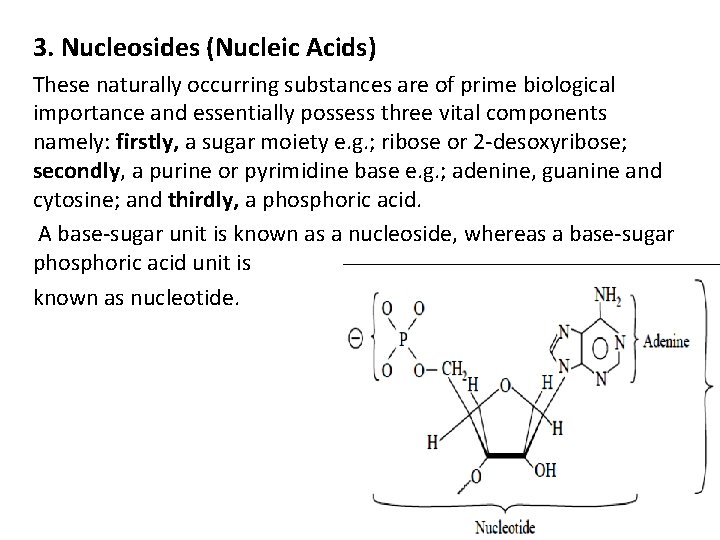
3. Nucleosides (Nucleic Acids) These naturally occurring substances are of prime biological importance and essentially possess three vital components namely: firstly, a sugar moiety e. g. ; ribose or 2 -desoxyribose; secondly, a purine or pyrimidine base e. g. ; adenine, guanine and cytosine; and thirdly, a phosphoric acid. A base-sugar unit is known as a nucleoside, whereas a base-sugar phosphoric acid unit is known as nucleotide.

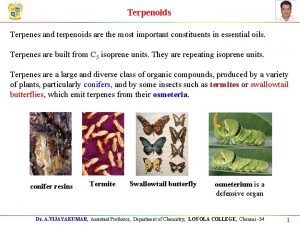
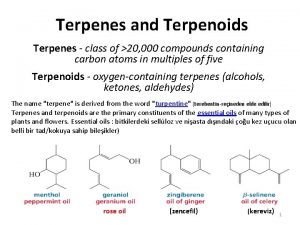
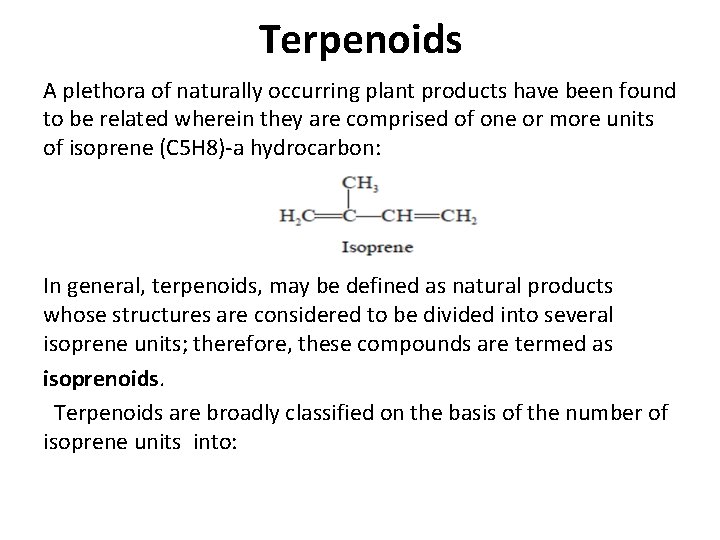
Terpenoids A plethora of naturally occurring plant products have been found to be related wherein they are comprised of one or more units of isoprene (C 5 H 8)-a hydrocarbon: In general, terpenoids, may be defined as natural products whose structures are considered to be divided into several isoprene units; therefore, these compounds are termed as isoprenoids. Terpenoids are broadly classified on the basis of the number of isoprene units into:

(a) Monoterpenoids: two isoprene units and have the molecular formula C 10 H 16. (b) Sesquiterpenoids: three isoprene units and have the molecular formula C 15 H 24. (c) Diterpenoids: four isoprene units and have the molecular formula C 20 H 32. (d) Triterpenoids: six isoprene units and have the molecular formula C 30 H 48. (e) Tetraterpenoids(or Carotenoids): : eight isoprene units and have the molecular formula C 40 H 64.


CLASSIFICATION (i) Monoterpenoids (ii) Sesquiterpenoids (iii) Diterpenoids (iv) Triterpenoids (v) Tetraterpenoids and Carotenoids (vi) Volatile Oils (or Essential Oils) (vii) Resins and Resin Combinations (viii) Oleoresins (ix) Oleo-Gum-Resins (x) Balsams

Resins and Resin Combinations • Resins, in general, are amorphous solid or semisolid substances that are invariably water insoluble but mostly soluble in alcohol or other organic solvents. • Physical Properties of Resins: 1. Are hard, transparent or translucent brittle materials. 2. They are invariably heavier than water having the specific gravity ranging from 0. 9 -1. 25. 3. On being heated at a relatively low temperature resins first get softened and ultimately melt by forming a sticky massive fluid, without undergoing any decomposition or volatilization.

4. On being heated in the air i. e. , in the presence of oxygen, resins usually burn readily with a smoky flame by virtue of the presence of a large number of C-atoms in their structure. 5. They are practically insoluble in water, but frequently soluble in ethanol, volatile oils, fixed oils, chloral hydrate and non-polar organic solvents e. g. , benzene, n-hexane and petroleum ether. _________________________ Chemical Properties of Resins: 1. Resins, in general, are enriched with carbon, and contain a few oxygen in their respective molecules. 2. Majority of them undergo slow atmospheric oxidation and their colour get darkened with impaired solubility. 3. Resins are found to be a mixture of numerous compounds rather than a single pure chemical entity.

Chemical Composition of Resins: (i) Resin Acids. (ii) Resin Esters and their Decomposition Products i. e. , Resin Alcohols (Resinols) and Resin Phenols (Resinotannols). (iii) Resenes i. e. , the chemical inert compounds.