Germline testing Susan Domchek MD University of Pennsylvania











- Slides: 21

Germline testing Susan Domchek, MD University of Pennsylvania

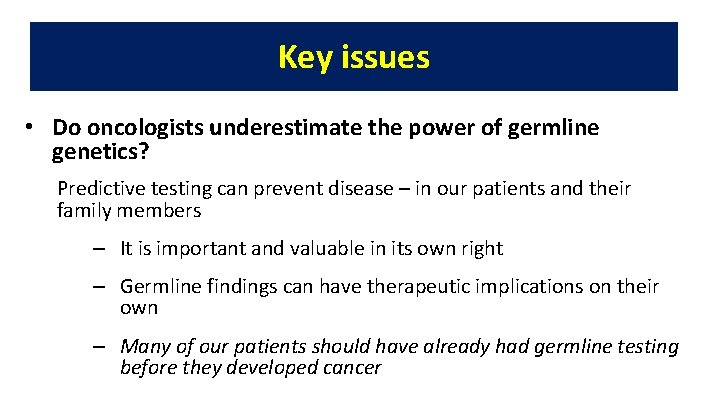
Key issues • Do oncologists underestimate the power of germline genetics? Predictive testing can prevent disease – in our patients and their family members – It is important and valuable in its own right – Germline findings can have therapeutic implications on their own – Many of our patients should have already had germline testing before they developed cancer

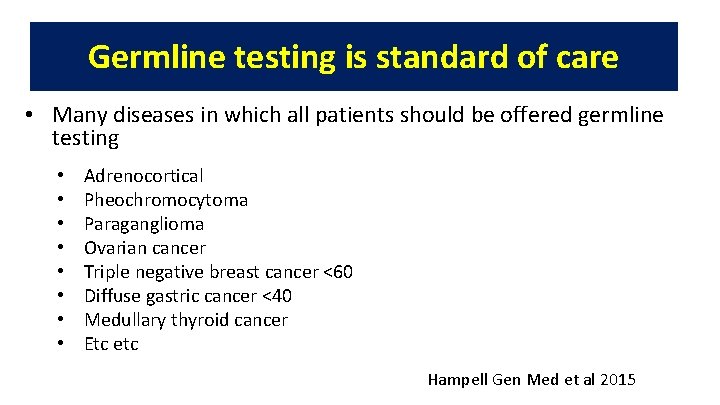
Germline testing is standard of care • Many diseases in which all patients should be offered germline testing • • Adrenocortical Pheochromocytoma Paraganglioma Ovarian cancer Triple negative breast cancer <60 Diffuse gastric cancer <40 Medullary thyroid cancer Etc etc Hampell Gen Med et al 2015

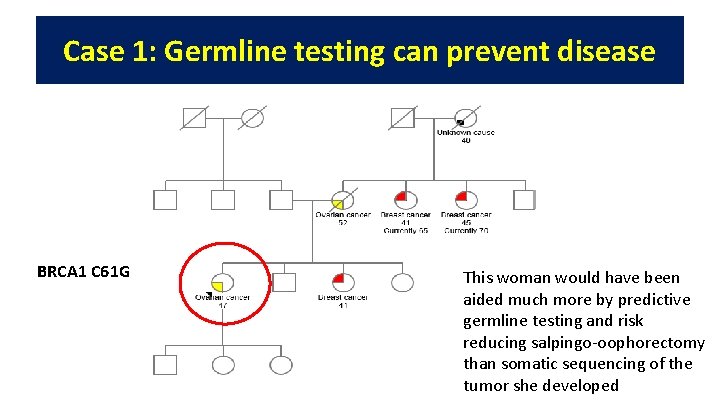
Case 1: Germline testing can prevent disease BRCA 1 C 61 G This woman would have been aided much more by predictive germline testing and risk reducing salpingo-oophorectomy than somatic sequencing of the tumor she developed

Key issues • There are several ways to do tumor testing: do patients and providers understand germline implications of each method? – Paired germline/somatic vs somatic with germline subtracted vs somatic only – Understand strengths and weaknesses – Develop strategies so that data can be used – Appropriate consent

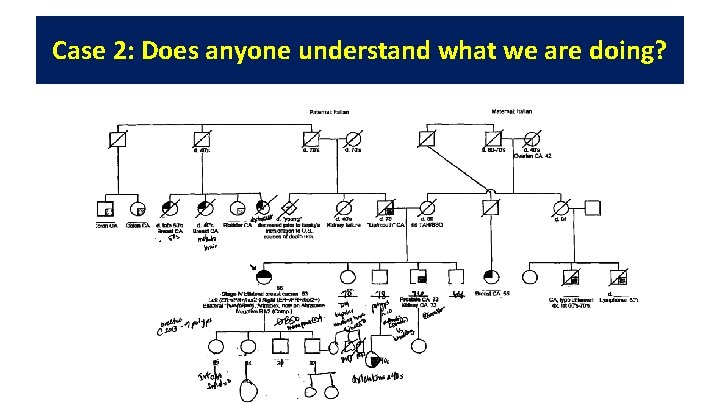
Case 2: Does anyone understand what we are doing?




Technical aspects of germline sequencing • Rationale for germline sequencing are different and sensitivity for mutations is not necessarily the same – Identification of cancer susceptibility variants vs. comparison to a tumor sequence – Sensitivity of deletion/duplication? Uniform coverage? – Current standard remains to do germline testing if indicated regardless of somatic results

Key issues • Reporting: – Differential level of variant review and annotation for germline and somatic – Testing is done for different reasons – and may have different thresholds – Not everything needs to be further evaluated in the germline (VUS/benign in Clin. Var) – Clarification of reporting • Founder mutations • Allelic frequency? • Beyond cancer related genes?

Case 3: Multiple reporting confusions • Oncologist at Penn had received germline and somatic testing reports and “it is very weird but the patient has different mutations in germline and tumor” – BRCA 1 C 61 G, c. 181 T>G: They are the same • Patient – very concerned because her uncle has a different germline mutation than she does – does she need to be retested? – BRCA 1 187 del. AG (also known as BRCA 1 185 del. AG and c. 68_69 del. AG) – Common Ashkenazi Jewish founder mutation



Case 4: Variants of Uncertain Significance • Not infrequent that findings on somatic reports are “unimportant” in Clin. Var – VUS: When found in the germline – we do not act on these, management is based on family history – GENIE and other initiatives – May be particularly important in loss of function – For alternations that on the germline side would be VUS at best – this may “dilute” the study, as we may not expect responses

Key issues • Somatic only – the de facto standard in the community at this time – What results should trigger rapid and low barrier referral for germline testing? Why does this matter? – Extending beyond the traditional model - cancer community has create new models

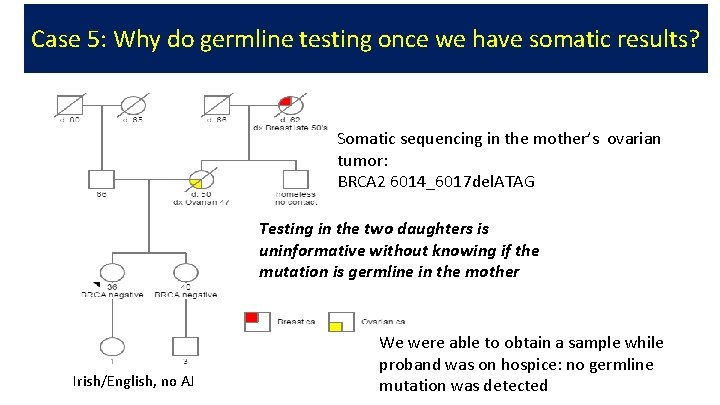
Case 5: Why do germline testing once we have somatic results? Somatic sequencing in the mother’s ovarian tumor: BRCA 2 6014_6017 del. ATAG Testing in the two daughters is uninformative without knowing if the mutation is germline in the mother Irish/English, no AJ We were able to obtain a sample while proband was on hospice: no germline mutation was detected

Best practice list: Somatic sequencing findings with consideration for gemline testing • Tumors with a pathogenic or likely pathogenic BRCA 1, BRCA 2, MLH 1, MSH 2, or MSH 6 mutations • Pathogenic variants in BRIP 1, PALB 2, RAD 51 C, RAD 51 D • Founder mutations (which will be specified in report) • TP 53 R 337 H; ATM V 2424 G; • APC I 1307 K; CHEK 2 1100 del. C, I 157 T, S 428 F will have explanatory text (as moderate prevalence founder/common mutations) • Pathogenic variants in genes for which your patient appears to have an associated clinical phenotype (See checklist ) • Patients whose personal or family history is suggestive of a hereditary cancer syndrome should be referred regardless of somatic testing results D Clark, K Maxwell, K Nathanson, S Domchek, J Morrissette

Considerations • Embrace the germline: risk prediction and prevention for patients and family members – as well as targeted therapy • Consent, disclosure, and data usage • Clarity of testing and reporting for providers and patients • Novel models for consent and information dissemination

Suggestions from the clinical oncologist 1. Encourage use of germline testing early in disease in appropriate candidates 2. Clarify reporting: Type of testing and reporting of results 3. – – – Clearly identify type of testing Clearly identify founder mutations Develop consistent reporting across germline and somatic sequencing Develop a best practice list of which somatic results should be promptly referred to germline testing

Recommendations from the germline panel 1. Encourage analysis of the germline 2. Improve annotation and reporting 3. Evolve paradigms for consent and return of results 4. Provide guidance for community oncologist for inferences of germline in somatic testing
 Susan domchek md
Susan domchek md Hanifin rajka atopic dermatitis
Hanifin rajka atopic dermatitis California university of pennsylvania global online
California university of pennsylvania global online Susan norton augusta university
Susan norton augusta university Commercial law rmit
Commercial law rmit Domain testing in software testing methodologies
Domain testing in software testing methodologies Logic based testing in software testing
Logic based testing in software testing Du path testing
Du path testing Positive testing and negative testing
Positive testing and negative testing Cs3250
Cs3250 Anuj magazine
Anuj magazine Functional testing vs unit testing
Functional testing vs unit testing Cause effect graphing technique
Cause effect graphing technique Control structure testing in software engineering
Control structure testing in software engineering Decision table testing in software testing
Decision table testing in software testing Decision table for triangle problem
Decision table for triangle problem Makalah black box testing
Makalah black box testing Black-box testing disebut juga sebagai behavioral testing
Black-box testing disebut juga sebagai behavioral testing Extended decision table
Extended decision table Rigorous testing in software testing
Rigorous testing in software testing Testing blindness in software testing
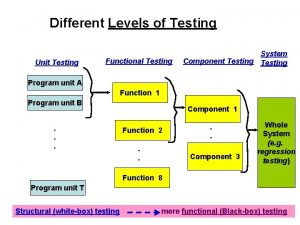
Testing blindness in software testing Component testing is a black box testing
Component testing is a black box testing