Future HIV Prevention Trials Sexual Transmission Willard Cates












- Slides: 24

Future HIV Prevention Trials: Sexual Transmission Willard Cates, Jr. , MD, MPH Family Health International 3 rd IAS Conference Rio de Janeiro July 26, 2005

Key Topics • HIV prevention tools • Current trials • Future opportunities • Future challenges

HIV Prevention Research - Why • Continued spread of HIV • Clear moral imperative to conduct prevention science

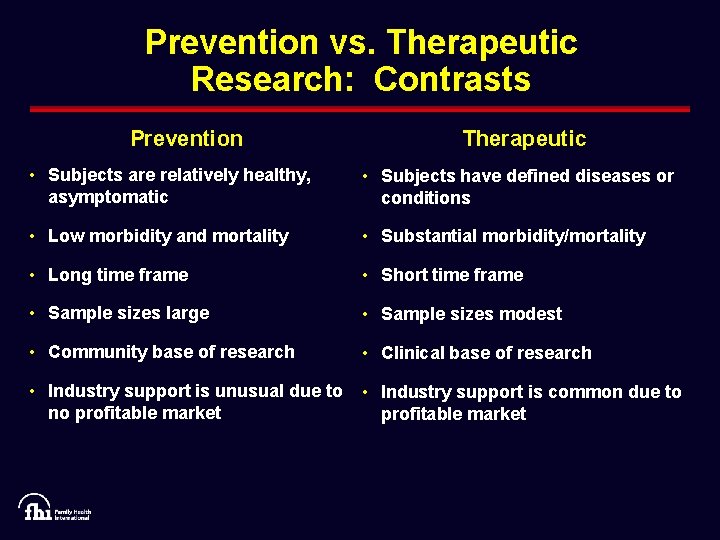
Prevention vs. Therapeutic Research: Contrasts Prevention Therapeutic • Subjects are relatively healthy, asymptomatic • Subjects have defined diseases or conditions • Low morbidity and mortality • Substantial morbidity/mortality • Long time frame • Short time frame • Sample sizes large • Sample sizes modest • Community base of research • Clinical base of research • Industry support is unusual due to no profitable market • Industry support is common due to profitable market

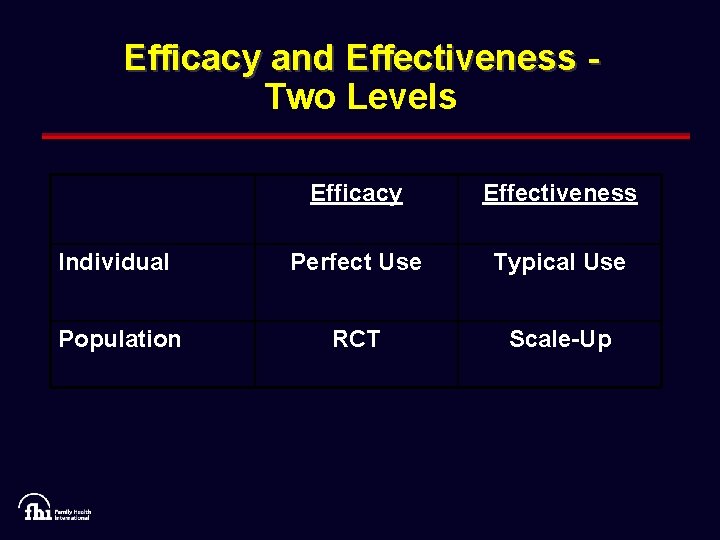
Efficacy and Effectiveness Two Levels Efficacy Effectiveness Individual Perfect Use Typical Use Population RCT Scale-Up

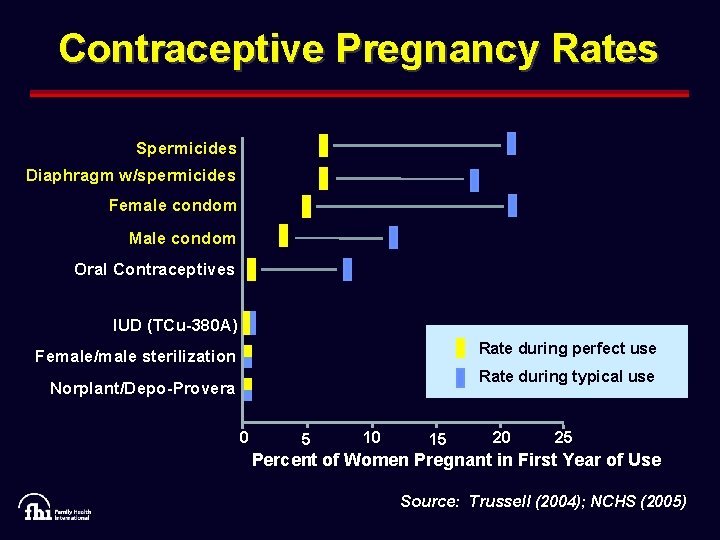
Contraceptive Pregnancy Rates Spermicides Diaphragm w/spermicides Female condom Male condom Oral Contraceptives IUD (TCu-380 A) Rate during perfect use Female/male sterilization Rate during typical use Norplant/Depo-Provera 0 5 10 15 20 25 Percent of Women Pregnant in First Year of Use Source: Trussell (2004); NCHS (2005)

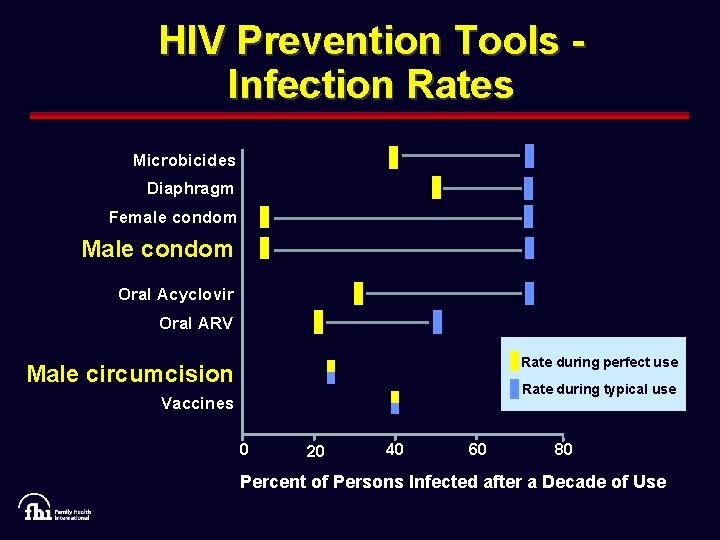
HIV Prevention Tools Infection Rates Microbicides Diaphragm Female condom Male condom Oral Acyclovir Oral ARV Rate during perfect use Male circumcision Rate during typical use Vaccines 0 20 40 60 80 Percent of Persons Infected after a Decade of Use

Topical Microbicides – 2 nd Generation Products – – – Buffergel * Pro 2000 C 31 G * Cellulose sulfate Carraguard * May also protect against pregnancy

Diaphragm • RCT in Zimbabwe, RSA • Diaphragm plus lubricant gel vs. condom • 4, 500 participants planned, 86% enrolled • Results expected 2007

Female Condoms • Biologic plausibility similar to male condoms • If made available, lowers overall level of unprotected acts • Emerging evidence (’ 05 ISSTDR) on STD prevention

Oral Acyclovir Prophylaxis • Acquisition – HSV+, HIV- subjects – HPTN 039 – USA and global – 3, 000 participants – MSM, MSW – Results expected 2007 • Transmission – HSV+, HIV+ subjects – Gates – US and global – 3, 600 HIV discordant couples – Results expected 2008

Oral ARV Pre-exposure Prophylaxis • Tenofovir collaboration – researchers and stakeholders • Focus for prevention trial ethics • Multiple populations globally • Estimated cumulative 4000 participants • Incremental results from summer 2006

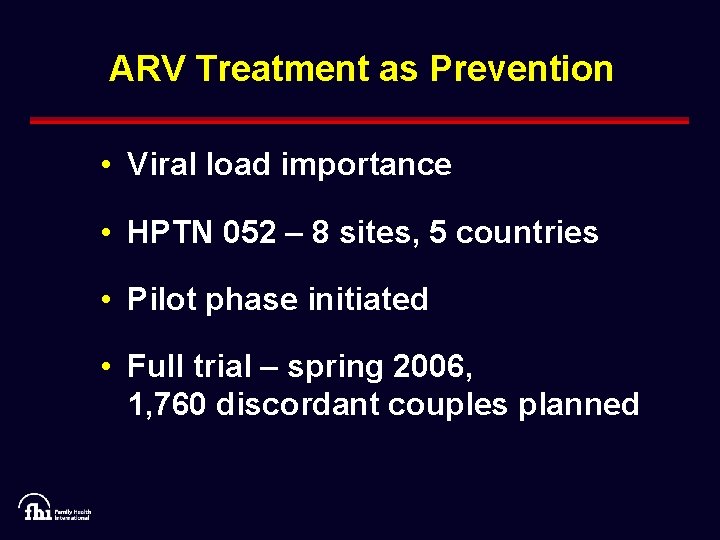
ARV Treatment as Prevention • Viral load importance • HPTN 052 – 8 sites, 5 countries • Pilot phase initiated • Full trial – spring 2006, 1, 760 discordant couples planned

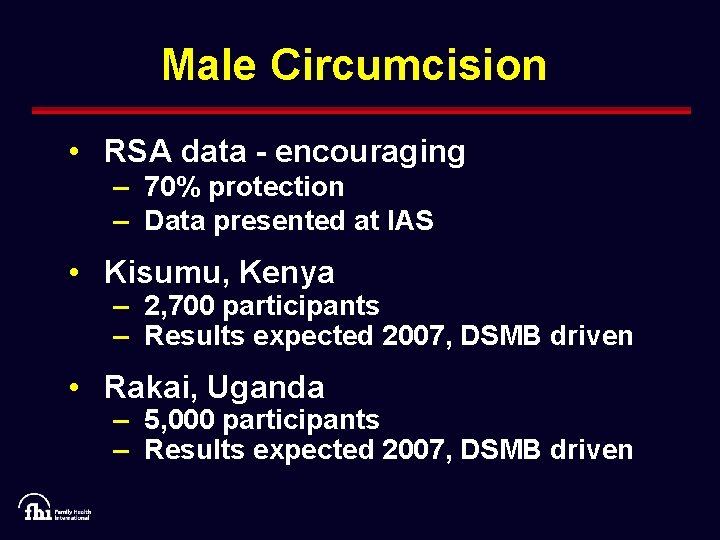
Male Circumcision • RSA data - encouraging – 70% protection – Data presented at IAS • Kisumu, Kenya – 2, 700 participants – Results expected 2007, DSMB driven • Rakai, Uganda – 5, 000 participants – Results expected 2007, DSMB driven

Future Directions • 3 rd generation microbicides • Newly designed barriers • Combination ARV pre-exposure • Acute HIV infection

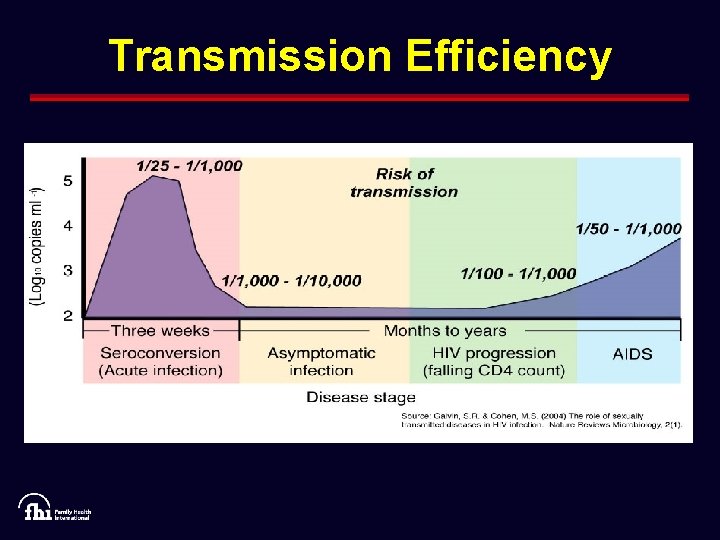
Transmission Efficiency

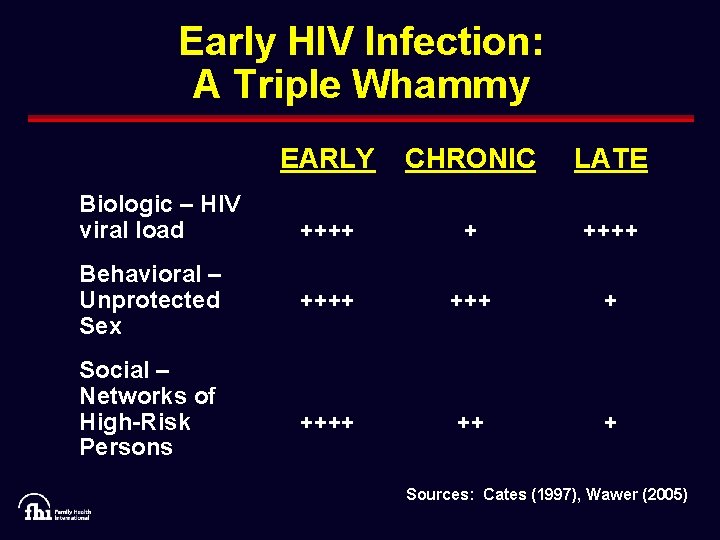
Early HIV Infection: A Triple Whammy Biologic – HIV viral load Behavioral – Unprotected Sex Social – Networks of High-Risk Persons EARLY CHRONIC LATE ++++ + ++++ ++ + Sources: Cates (1997), Wawer (2005)

Future Challenges • Participant care/treatment • Standards of preventive care • Community engagement • Product interruption • Additional study sites

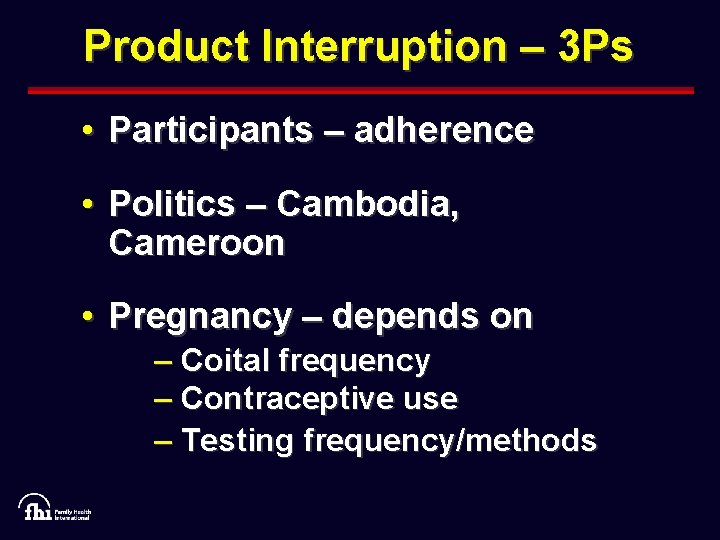
Product Interruption – 3 Ps • Participants – adherence • Politics – Cambodia, Cameroon • Pregnancy – depends on – Coital frequency – Contraceptive use – Testing frequency/methods

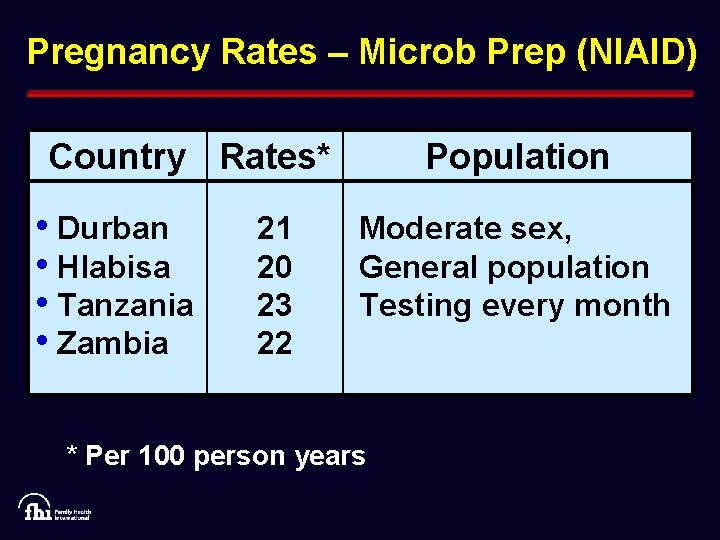
Pregnancy Rates – Microb Prep (NIAID) Country Rates* • Durban • Hlabisa • Tanzania • Zambia 21 20 23 22 Population Moderate sex, General population Testing every month * Per 100 person years

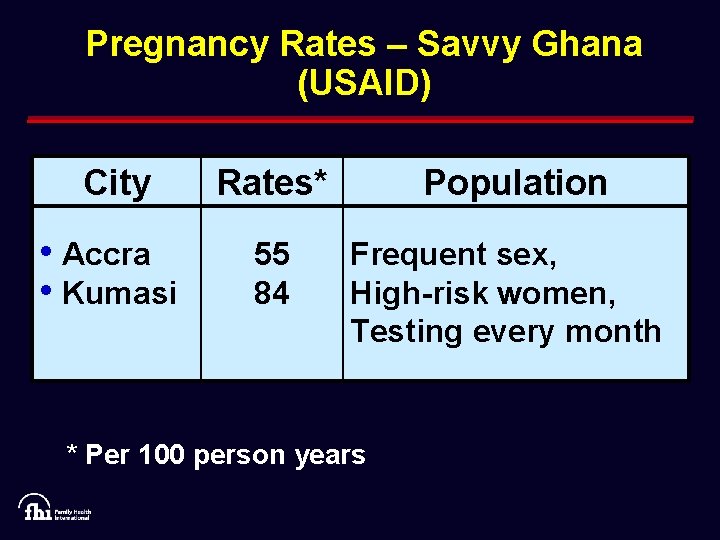
Pregnancy Rates – Savvy Ghana (USAID) City Rates* Population • Accra • Kumasi 55 84 Frequent sex, High-risk women, Testing every month * Per 100 person years

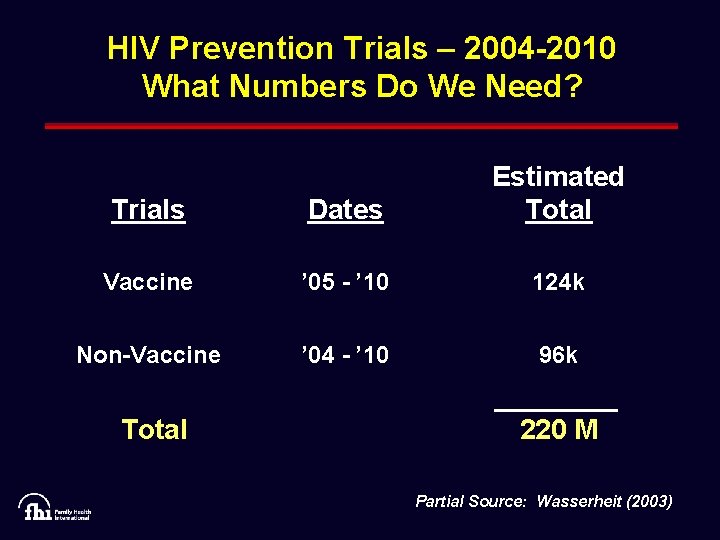
HIV Prevention Trials – 2004 -2010 What Numbers Do We Need? Trials Dates Estimated Total Vaccine ’ 05 - ’ 10 124 k Non-Vaccine ’ 04 - ’ 10 96 k Total 220 M Partial Source: Wasserheit (2003)

Conclusion – HIV Prevention Trials, 2005 -2010 • Current Phase IIIs essential • More site capacity needed • Collaboration with other fields useful – vaccine, circumcision, STD rx, etc. • Shared resources helpful - GCP training - Laboratory assessment - Research staff - Ethics standards - Community involvement - Design challenges

Thanks To • Judy Auerbach • Nancy Padian • Michael Cassell • Renee Ridzon • Mike Cohen • Ken Schulz • Helene Gayle • James Trussell • Ron Gray • Sten Vermund • King Holmes • Judy Wasserheit • Cynthia Kay • Gary West
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Stakeholders in hiv prevention
Stakeholders in hiv prevention Global hiv prevention coalition
Global hiv prevention coalition Jared cates
Jared cates Nick cates design
Nick cates design Sexual harrasment prevention training
Sexual harrasment prevention training Future perfect simple continuous
Future perfect simple continuous Future continuous and future perfect simple
Future continuous and future perfect simple Futuresearch trials
Futuresearch trials Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Willard mwemba
Willard mwemba Dallas willard healing the heart
Dallas willard healing the heart Willard wigan mona lisa
Willard wigan mona lisa Traços cardinais
Traços cardinais Willard scott birthday announcement form
Willard scott birthday announcement form Future vacation
Future vacation Nulti kondicional
Nulti kondicional Future continuous future perfect exercises
Future continuous future perfect exercises Tenses in english
Tenses in english Future nurse future midwife e learning
Future nurse future midwife e learning Future plans and finished future actions
Future plans and finished future actions Future perfect interrogative
Future perfect interrogative Present progressive with future meaning examples
Present progressive with future meaning examples