FUSION Susan Cartwright Nuclear Physics Atoms consist of




- Slides: 13

FUSION Susan Cartwright

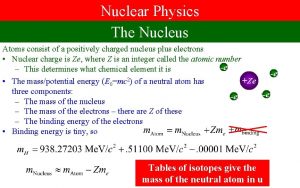
Nuclear Physics • Atoms consist of a nucleus surrounded by electrons • Chemistry is produced by interactions involving the electrons around the atom • this involves small energy changes (~0. 01 e. V) and can happen at room temperatures • our lives are dominated by chemical reactions • Nuclear physics is concerned with reactions of the atomic nuclei • these involve much larger energies (~Me. V) and therefore normally happen at high temperatures • we are not so aware of nuclear reactions in our everyday lives, but they are critical to our existence

Why is nuclear physics important? • Naturally occurring nuclear reactions are crucial to our very existence • They power the Sun • They created most of the elements in your body (the exception being hydrogen) • Engineered nuclear reactions can also be useful • Medical applications • radiotherapy, PET scanners • Nuclear fission as a power source • much safer than most people think it is, and contributes less to global warming • Nuclear fusion as a power source? • “always 50 years away”?

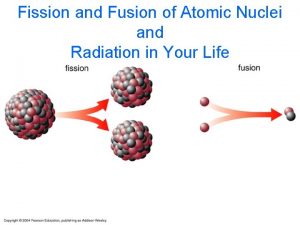
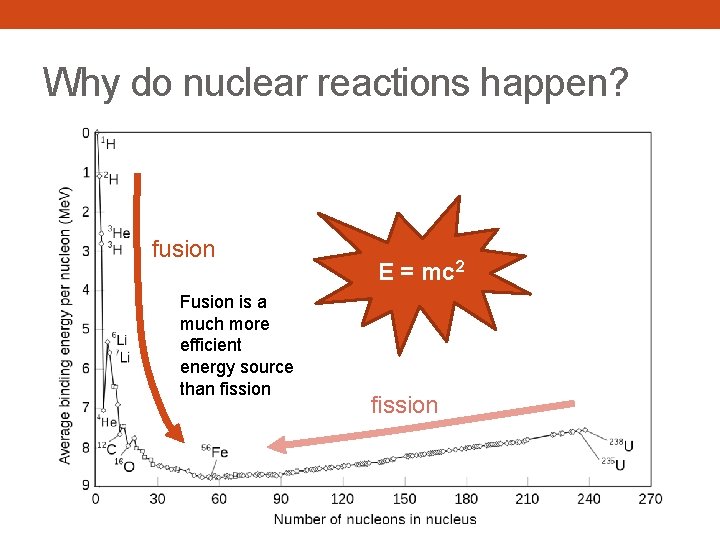
Why do nuclear reactions happen? fusion Fusion is a much more efficient energy source than fission E = mc 2 fission

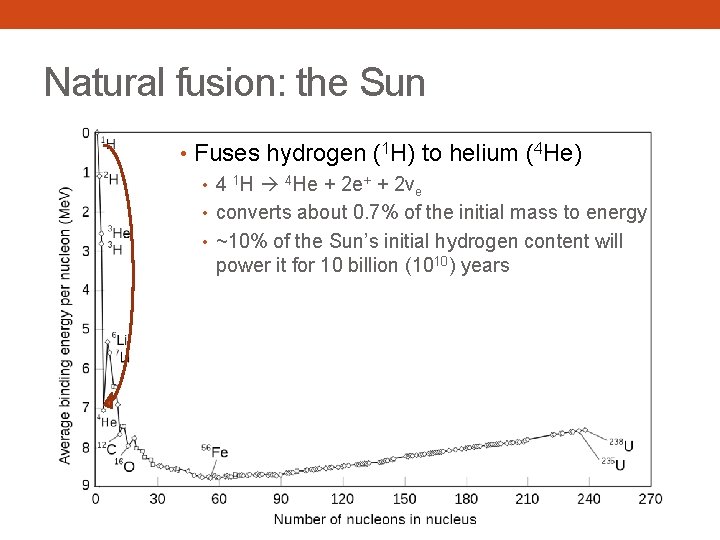
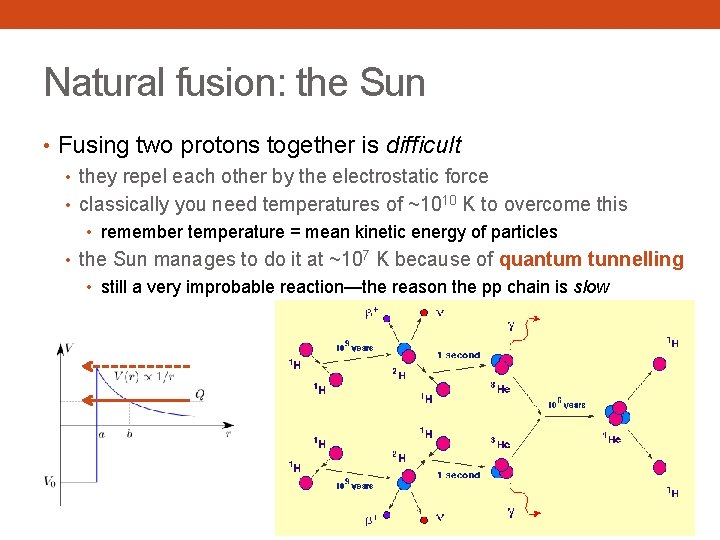
Natural fusion: the Sun • Fuses hydrogen (1 H) to helium (4 He) • 4 1 H 4 He + 2 e+ + 2νe • converts about 0. 7% of the initial mass to energy • ~10% of the Sun’s initial hydrogen content will power it for 10 billion (1010) years

Natural fusion: the Sun • Fusing two protons together is difficult • they repel each other by the electrostatic force • classically you need temperatures of ~1010 K to overcome this • remember temperature = mean kinetic energy of particles • the Sun manages to do it at ~107 K because of quantum tunnelling • still a very improbable reaction—the reason the pp chain is slow

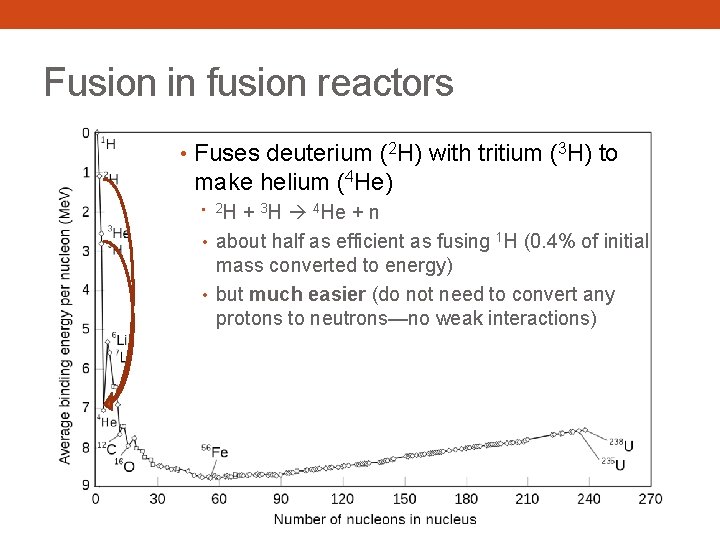
Fusion in fusion reactors • Fuses deuterium (2 H) with tritium (3 H) to make helium (4 He) H + 3 H 4 He + n • about half as efficient as fusing 1 H (0. 4% of initial mass converted to energy) • but much easier (do not need to convert any protons to neutrons—no weak interactions) • 2

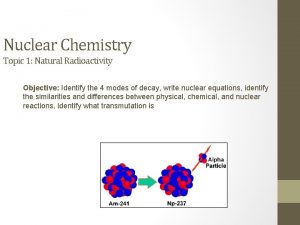
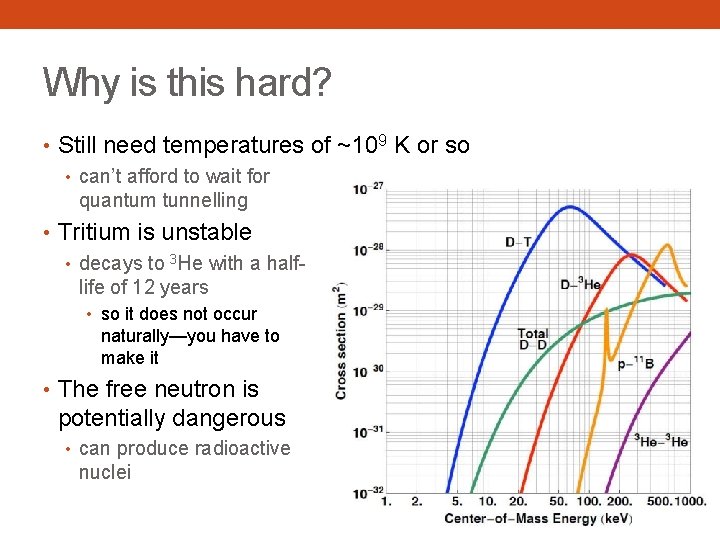
Why is this hard? • Still need temperatures of ~109 K or so • can’t afford to wait for quantum tunnelling • Tritium is unstable • decays to 3 He with a halflife of 12 years • so it does not occur naturally—you have to make it • The free neutron is potentially dangerous • can produce radioactive nuclei

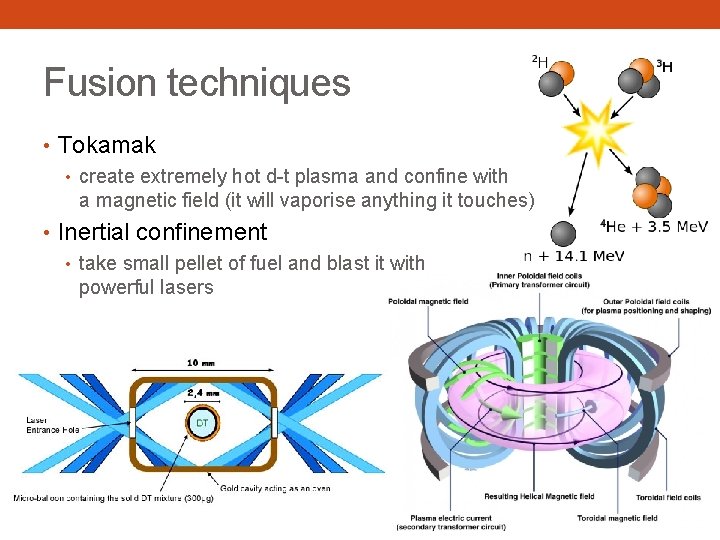
Fusion techniques • Tokamak • create extremely hot d-t plasma and confine with a magnetic field (it will vaporise anything it touches) • Inertial confinement • take small pellet of fuel and blast it with powerful lasers

The ITER fusion reactor

National Ignition Facility

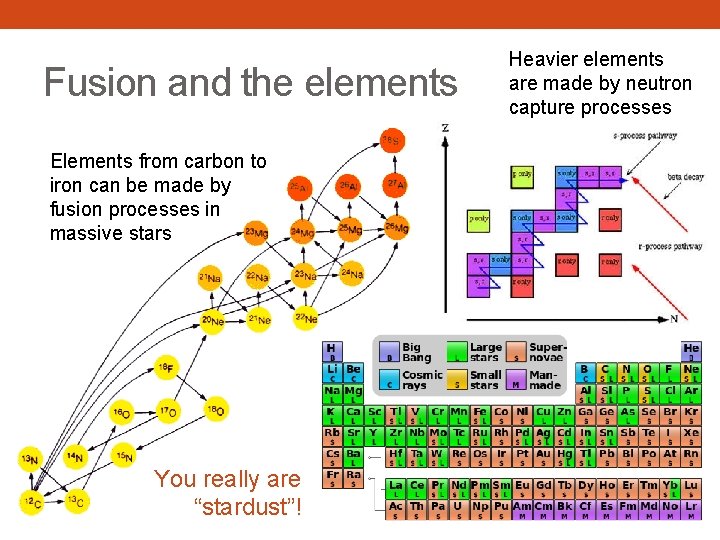
Fusion and the elements Elements from carbon to iron can be made by fusion processes in massive stars You really are “stardust”! Heavier elements are made by neutron capture processes

Conclusions • Fusion is a promising future energy source • next generation fusion reactors are coming close to “break-even” • But there are issues • tritium supply is an issue • need to contain the free neutrons • Clever idea: line tokamak with lithium • neutron capture on 6 Li makes tritium: 6 Li + n 4 He + 3 H • remove neutrons and regenerate tritium! • such “lithium blankets” are a current focus of research • Meanwhile, small-scale d-t fusion is used as a convenient source of neutrons for research • as is the case here in Sheffield…
 Who discovered neutrino
Who discovered neutrino Susan cartwright sheffield
Susan cartwright sheffield Dr susan cartwright
Dr susan cartwright Susan cartwright sheffield
Susan cartwright sheffield Susan cartwright sheffield
Susan cartwright sheffield At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Are nuclear power plants fission or fusion
Are nuclear power plants fission or fusion Fusion equation example
Fusion equation example Fission vs fusion nuclear
Fission vs fusion nuclear Nuclear fusion
Nuclear fusion Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Fission v fusion
Fission v fusion Nuclear fusion radiation
Nuclear fusion radiation Natural and artificial radioactivity
Natural and artificial radioactivity