Fluid Interface Atomic Force Microscopy FIAFM D Eric

- Slides: 12

Fluid Interface Atomic Force Microscopy (FI-AFM) D. Eric Aston Prof. John C. Berg, Advisor Department of Chemical Engineering University of Washington

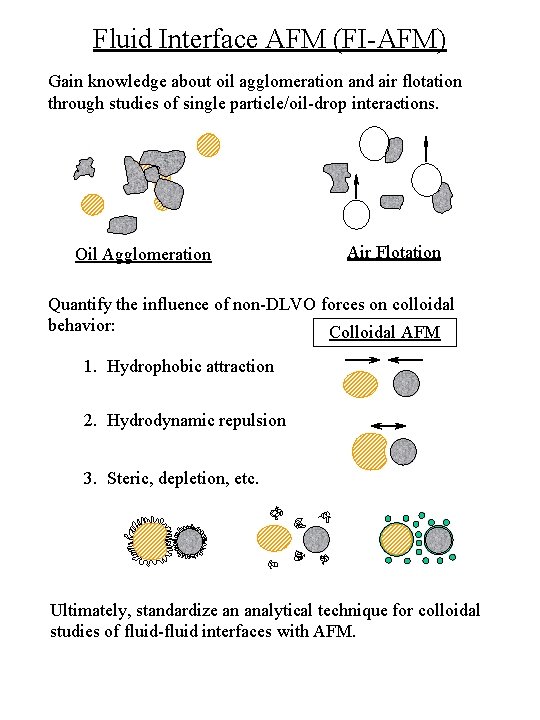
Fluid Interface AFM (FI-AFM) Gain knowledge about oil agglomeration and air flotation through studies of single particle/oil-drop interactions. Oil Agglomeration Air Flotation Quantify the influence of non-DLVO forces on colloidal behavior: Colloidal AFM 1. Hydrophobic attraction 2. Hydrodynamic repulsion 3. Steric, depletion, etc. Ultimately, standardize an analytical technique for colloidal studies of fluid-fluid interfaces with AFM.

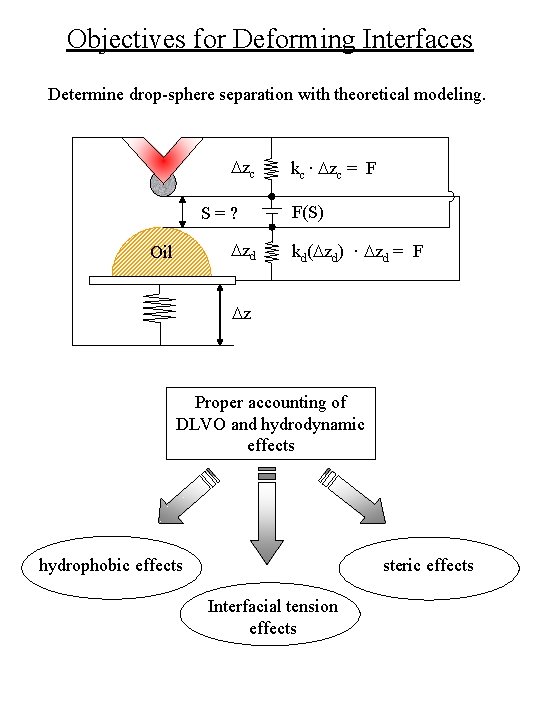
Objectives for Deforming Interfaces Determine drop-sphere separation with theoretical modeling. Dzc S=? Dzd Oil kc · Dzc = F F(S) kd(Dzd) · Dzd = F Dz Proper accounting of DLVO and hydrodynamic effects hydrophobic effects steric effects Interfacial tension effects

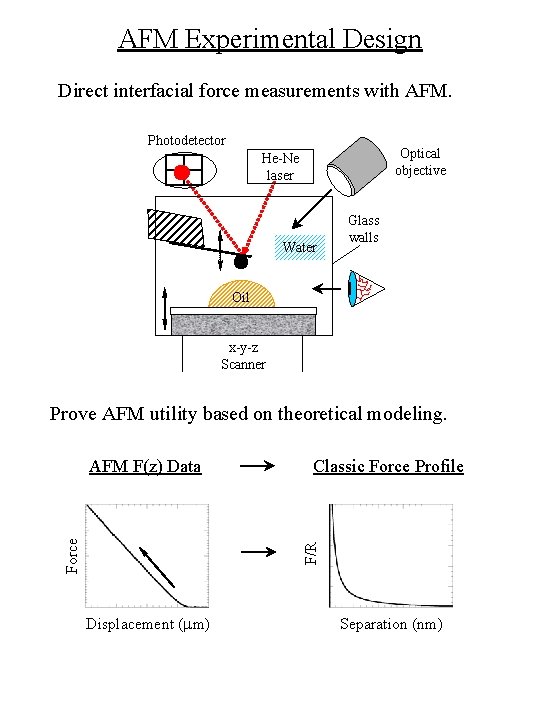
AFM Experimental Design Direct interfacial force measurements with AFM. Photodetector Optical objective He-Ne laser Water Glass walls Oil x-y-z Scanner Prove AFM utility based on theoretical modeling. Classic Force Profile F/R Force AFM F(z) Data Displacement (mm) Separation (nm)

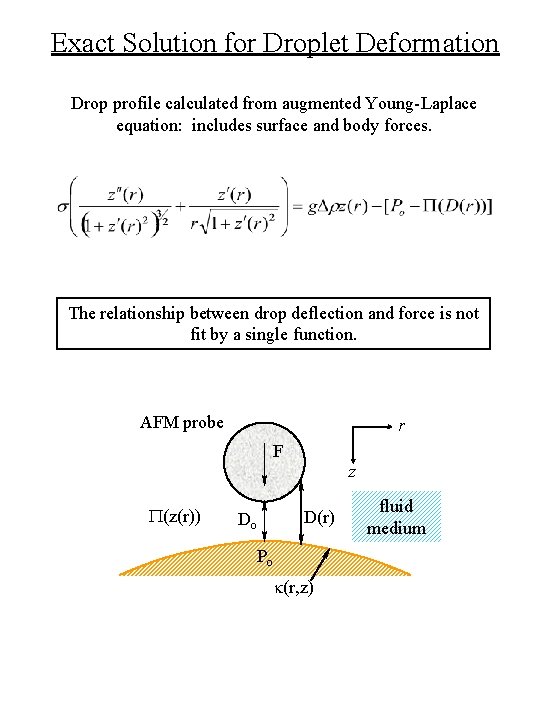
Exact Solution for Droplet Deformation Drop profile calculated from augmented Young-Laplace equation: includes surface and body forces. The relationship between drop deflection and force is not fit by a single function. AFM probe r F z P(z(r)) D(r) Do Po k(r, z) fluid medium

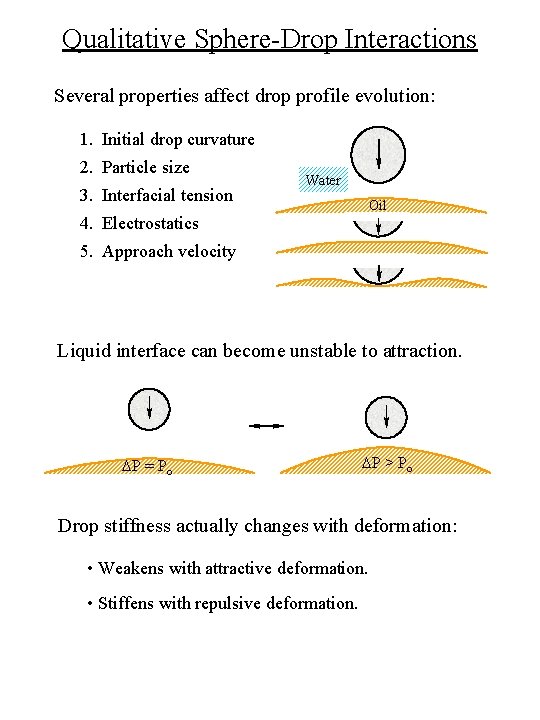
Qualitative Sphere-Drop Interactions Several properties affect drop profile evolution: 1. Initial drop curvature 2. Particle size 3. Interfacial tension Water Oil 4. Electrostatics 5. Approach velocity Liquid interface can become unstable to attraction. DP = Po DP > Po Drop stiffness actually changes with deformation: • Weakens with attractive deformation. • Stiffens with repulsive deformation.

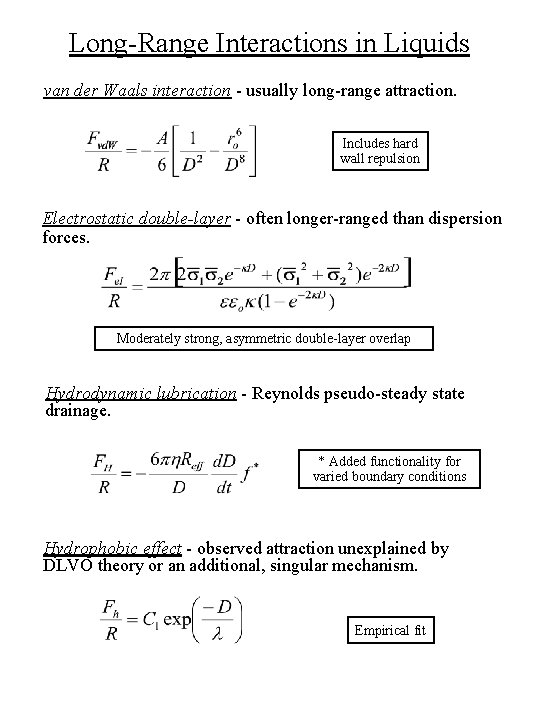
Long-Range Interactions in Liquids van der Waals interaction - usually long-range attraction. Includes hard wall repulsion Electrostatic double-layer - often longer-ranged than dispersion forces. Moderately strong, asymmetric double-layer overlap Hydrodynamic lubrication - Reynolds pseudo-steady state drainage. * Added functionality for varied boundary conditions Hydrophobic effect - observed attraction unexplained by DLVO theory or an additional, singular mechanism. Empirical fit

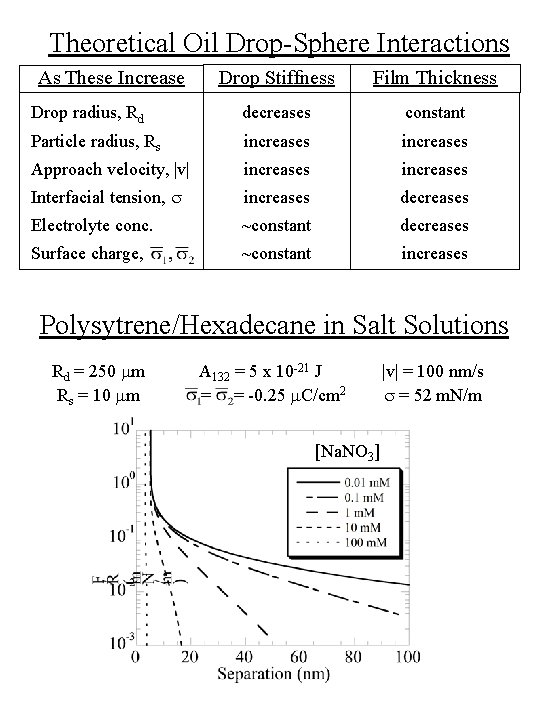
Theoretical Oil Drop-Sphere Interactions Drop Stiffness Film Thickness Drop radius, Rd decreases constant Particle radius, Rs increases Approach velocity, |v| increases Interfacial tension, s increases decreases Electrolyte conc. ~constant decreases Surface charge, ~constant increases As These Increase Polysytrene/Hexadecane in Salt Solutions Rd = 250 mm Rs = 10 mm A 132 = 5 x 10 -21 J = = -0. 25 m. C/cm 2 [Na. NO 3] |v| = 100 nm/s s = 52 m. N/m

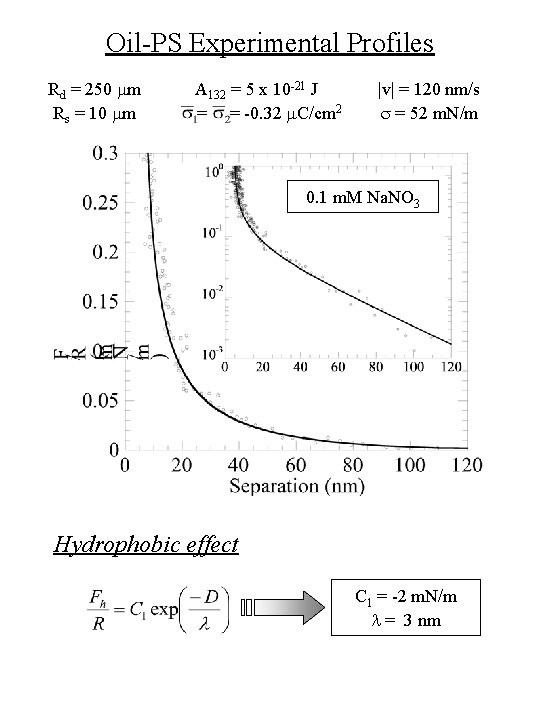
Oil-PS Experimental Profiles Rd = 250 mm Rs = 10 mm A 132 = 5 x 10 -21 J = = -0. 32 m. C/cm 2 |v| = 120 nm/s s = 52 m. N/m 0. 1 m. M Na. NO 3 Hydrophobic effect C 1 = -2 m. N/m l = 3 nm

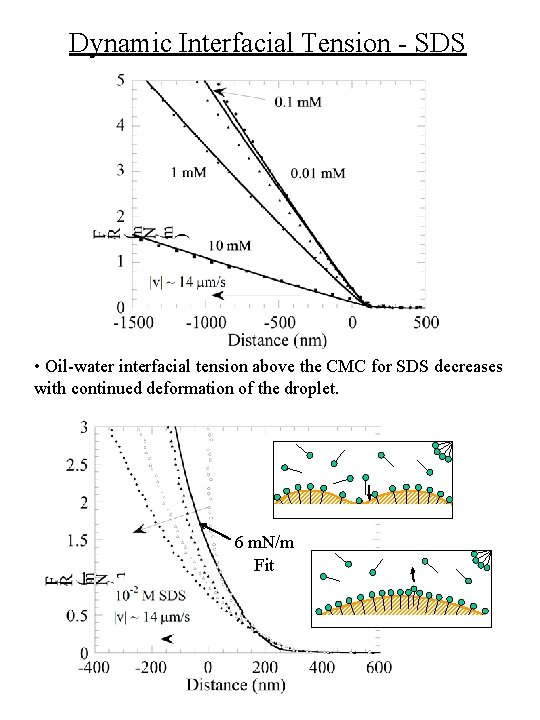
Dynamic Interfacial Tension - SDS • Oil-water interfacial tension above the CMC for SDS decreases with continued deformation of the droplet. 6 m. N/m Fit

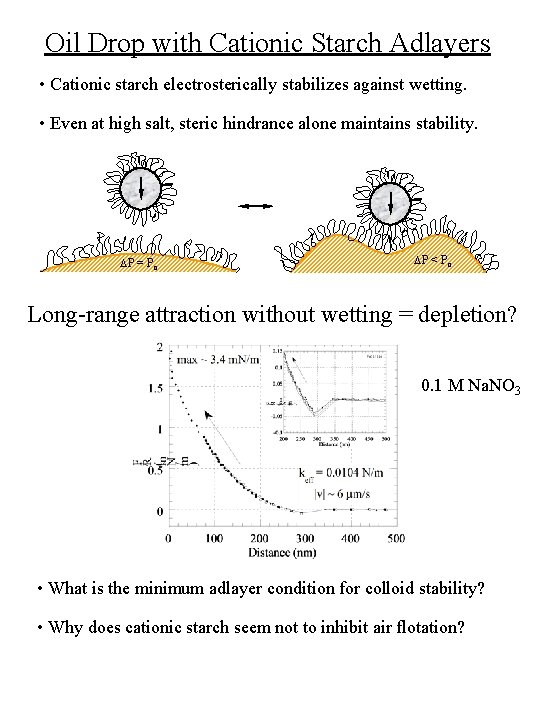
Oil Drop with Cationic Starch Adlayers • Cationic starch electrosterically stabilizes against wetting. • Even at high salt, steric hindrance alone maintains stability. DP = Po DP < Po Long-range attraction without wetting = depletion? 0. 1 M Na. NO 3 • What is the minimum adlayer condition for colloid stability? • Why does cationic starch seem not to inhibit air flotation?

Conclusions • Expectation of a dominant hydrophobic interaction is premature without thorough consideration of the deforming interface. • Several system parameters are key for interpreting fluid interfacial phenomena, all affecting drop deformation. 1. Surface forces - DLVO, hydrophobic, etc. 2. Drop and particle size - geometry of film drainage 3. Interfacial tension - promotion of film drainage 4. Approach velocity - resistance to film drainage • FI-AFM greatly expands our ability to explore fluid interfaces on an ideal scale.