FLIPPED CLASSROOM DESIGN FDP 201 x Pedagogy for











- Slides: 19

FLIPPED CLASSROOM DESIGN FDP 201 x: Pedagogy for Online and Blended Teaching-Learning Process Dr. SENTHILKUMAR SANTHANAMAHALINGAM This work is licensed under the Creative Commons Attribution-Share Alike 4. 0 International License. IDP in Educational Technology, IIT Bombay 1

Dr. SENTHILKUMAR SANTHANAMAHALINGAM About myself I have been working as an Assistant Professor in the Department of Chemistry, Ayya Nadar Janaki Ammal College (ANJAC), Sivakasi, Tamilnadu. I did my under-graduation, post-graduation and pre-doctoral degree from ANJAC, Sivakasi. I completed my Ph. D (full-time) from School of Chemistry, Madurai Kamaraj University, Madurai, Tamilnadu. I have about 7 years of research experience and 6 years of teaching experience. At present, I have been teaching chemistry, specialization in Inorganic Chemistry to the students form rural background. Most of the students are first generation learners. I have been motivating the students to enter into higher education especially to do research and serve for the nation. IDP in Educational Technology, IIT Bombay 2

Dr. SENTHILKUMAR SANTHANAMAHALINGAM TOPIC VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR) Theory COURSE CHEMICAL BONDING DOMAIN CHEMISTRY TARGET AUDIENCE FIRST YEAR UNDERGRADUATE CHEMISTRY STUDENTS AFFILIATION AYYA NADAR JANAKI AMMAL COLLEGE, SIVAKASI IDP in Educational Technology, IIT Bombay 3

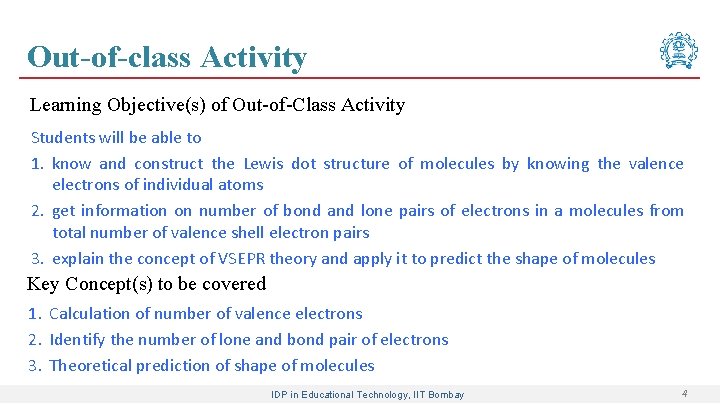
Out-of-class Activity Learning Objective(s) of Out-of-Class Activity Students will be able to 1. know and construct the Lewis dot structure of molecules by knowing the valence electrons of individual atoms 2. get information on number of bond and lone pairs of electrons in a molecules from total number of valence shell electron pairs 3. explain the concept of VSEPR theory and apply it to predict the shape of molecules Key Concept(s) to be covered 1. Calculation of number of valence electrons 2. Identify the number of lone and bond pair of electrons 3. Theoretical prediction of shape of molecules IDP in Educational Technology, IIT Bombay 4

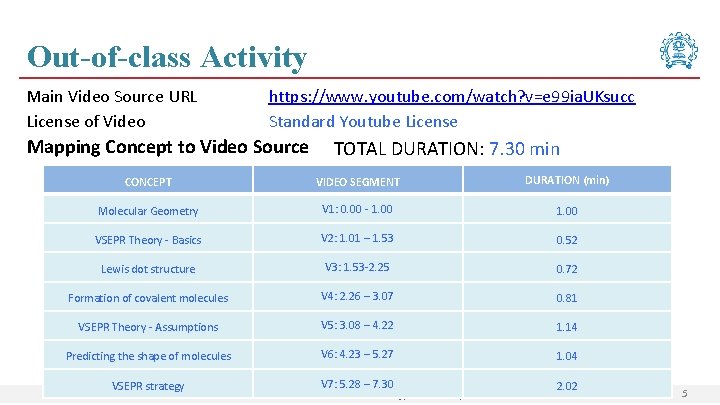
Out-of-class Activity Main Video Source URL License of Video https: //www. youtube. com/watch? v=e 99 ia. UKsucc Standard Youtube License Mapping Concept to Video Source TOTAL DURATION: 7. 30 min CONCEPT VIDEO SEGMENT DURATION (min) Molecular Geometry V 1: 0. 00 - 1. 00 VSEPR Theory - Basics V 2: 1. 01 – 1. 53 0. 52 Lewis dot structure V 3: 1. 53 -2. 25 0. 72 Formation of covalent molecules V 4: 2. 26 – 3. 07 0. 81 VSEPR Theory - Assumptions V 5: 3. 08 – 4. 22 1. 14 Predicting the shape of molecules V 6: 4. 23 – 5. 27 1. 04 V 7: 5. 28 – 7. 30 2. 02 VSEPR strategy IDP in Educational Technology, IIT Bombay 5

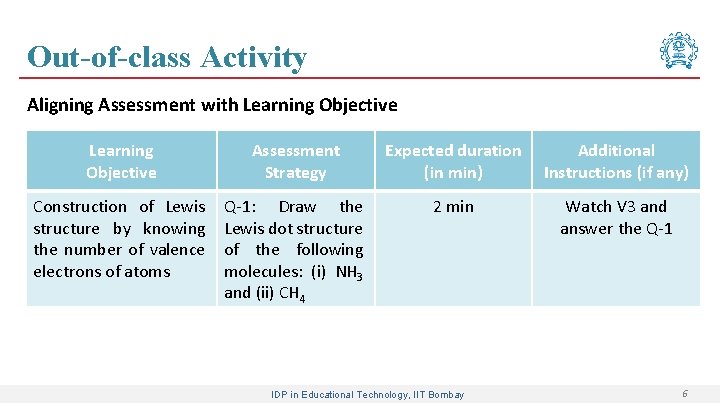
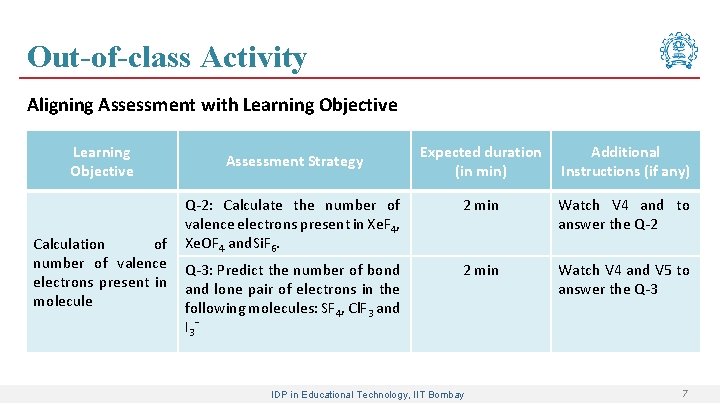
Out-of-class Activity Aligning Assessment with Learning Objective Assessment Strategy Expected duration (in min) Additional Instructions (if any) Construction of Lewis structure by knowing the number of valence electrons of atoms Q-1: Draw the Lewis dot structure of the following molecules: (i) NH 3 and (ii) CH 4 2 min Watch V 3 and answer the Q-1 IDP in Educational Technology, IIT Bombay 6

Out-of-class Activity Aligning Assessment with Learning Objective Calculation of number of valence electrons present in molecule Expected duration (in min) Additional Instructions (if any) Q-2: Calculate the number of valence electrons present in Xe. F 4, Xe. OF 4 and. Si. F 6. 2 min Watch V 4 and to answer the Q-2 Q-3: Predict the number of bond and lone pair of electrons in the following molecules: SF 4, Cl. F 3 and I 3 ˉ 2 min Watch V 4 and V 5 to answer the Q-3 Assessment Strategy IDP in Educational Technology, IIT Bombay 7

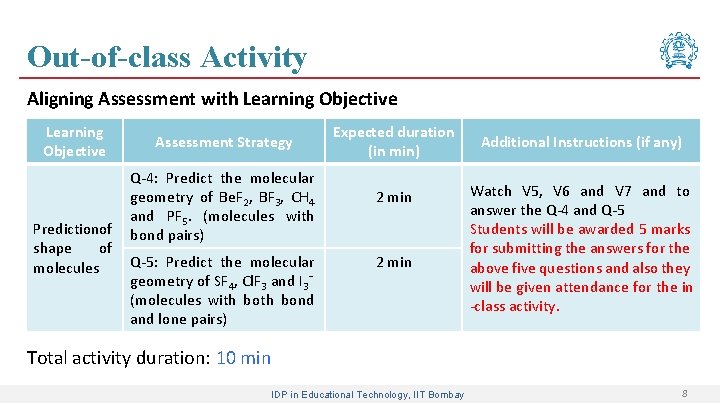
Out-of-class Activity Aligning Assessment with Learning Objective Predictionof shape of molecules Assessment Strategy Q-4: Predict the molecular geometry of Be. F 2, BF 3, CH 4 and PF 5. (molecules with bond pairs) Q-5: Predict the molecular geometry of SF 4, Cl. F 3 and I 3ˉ (molecules with bond and lone pairs) Expected duration (in min) 2 min Additional Instructions (if any) Watch V 5, V 6 and V 7 and to answer the Q-4 and Q-5 Students will be awarded 5 marks for submitting the answers for the above five questions and also they will be given attendance for the in -class activity. Total activity duration: 10 min IDP in Educational Technology, IIT Bombay 8

In-class Activity Learning Objective(s) of Out-of-Class Activity Students will be able to 1. create the problem solving skills through VSEPR theory 2. analyse the common mistakes while predicting the shape of molecules 3. Know the exceptional cases (molecules which do not obey VSEPR theory) Key Concept(s) to be covered 1. Problem solving skills for competitive examinations 2. To know about the exceptional cases 3. Common mistakes to be avoided IDP in Educational Technology, IIT Bombay 9

In-class Activity Active Learning Activities 1. Problem solving skills and common mistakes that students do through Think-Pair-Share 2. Clarifications and exceptions in VSEPR theory through Peer Instruction IDP in Educational Technology, IIT Bombay 10

In-class Activity Peer Instruction Strategy – What Teacher Does 1. Which of the following species has two non-bonded electron pairs on the central atom (a) Te. Cl 4 (b) Cl. F 3 (c) ICl 2ˉ (d) PCl 3 IDP in Educational Technology, IIT Bombay 11

In-class Activity Peer Instruction Strategy – What Teacher Does 2. Which of the following will have the molecular shape of a trigonal bipyramidal? (a) PF 3 Cl 2 (b) IF 5 (c) Br. F 5 (d) Sb. F 5ˉ IDP in Educational Technology, IIT Bombay 12

In-class Activity Peer Instruction Strategy – What Teacher Does 3. According to VSEPR model, the shape of [Xe. OF 5]ˉ is (a) Octahedral (b) trigonal bipyramidal (c) Square planar (d) pentagonal monopyramidal IDP in Educational Technology, IIT Bombay 13

In-class Activity Peer Instruction Strategy – What Student Does • Students are permitted to vote for the questions. • Students discussed with peers and come to consensus • Listened to instructors explanation IDP in Educational Technology, IIT Bombay 14

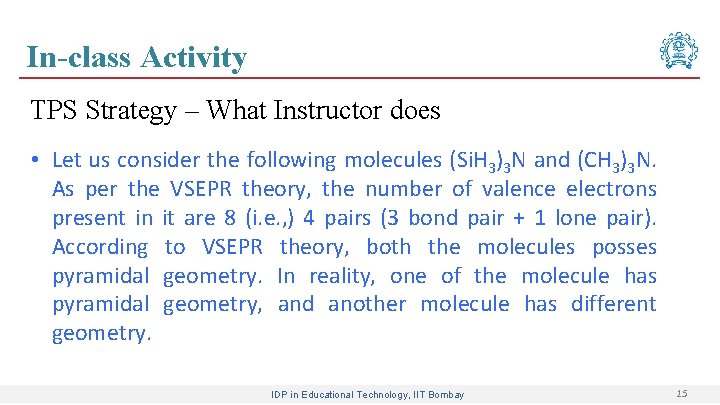
In-class Activity TPS Strategy – What Instructor does • Let us consider the following molecules (Si. H 3)3 N and (CH 3)3 N. As per the VSEPR theory, the number of valence electrons present in it are 8 (i. e. , ) 4 pairs (3 bond pair + 1 lone pair). According to VSEPR theory, both the molecules posses pyramidal geometry. In reality, one of the molecule has pyramidal geometry, and another molecule has different geometry. IDP in Educational Technology, IIT Bombay 15

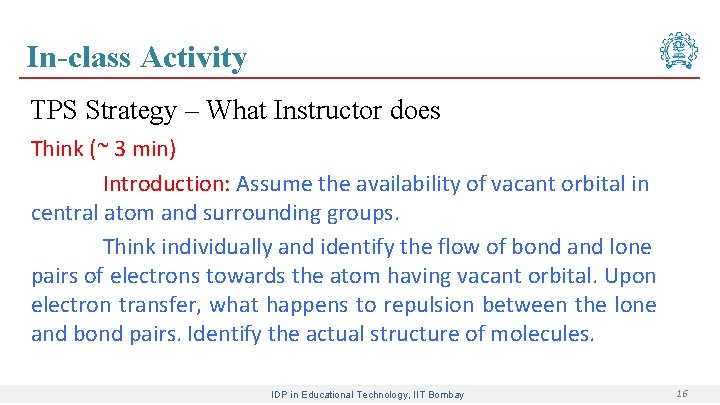
In-class Activity TPS Strategy – What Instructor does Think (~ 3 min) Introduction: Assume the availability of vacant orbital in central atom and surrounding groups. Think individually and identify the flow of bond and lone pairs of electrons towards the atom having vacant orbital. Upon electron transfer, what happens to repulsion between the lone and bond pairs. Identify the actual structure of molecules. IDP in Educational Technology, IIT Bombay 16

In-class Activity TPS Strategy – What Instructor does Pair (~ 5 min) Introduction: Students should pair up with your neighbour and come to the conclusion with individual structures of molecules. While pair phase, activities of 3 -4 groups have been monitored by instructor. Among the surrounding groups CH 3 and Si. H 3, Si. H 3 has vacant d-orbital in it. IDP in Educational Technology, IIT Bombay 17

In-class Activity TPS Strategy – What Instructor does Share (~ 8 min) Introduction: Students should form a group among your self and discuss for about 8 min. Students should come out with the correct answer (i. e. , ) correct structure of the (Si. H 3)3 N and (CH 3)3 N. Feedback from Instructor: Among the surrounding groups CH 3 and Si. H 3, Si. H 3 has vacant d-orbital in it. It result in the flow of lone pair of electrons on Natom to Si. H 3 group. Hence there is p-bond formation between the N-Si atoms. Since electron density (lone pair) from the N-atom has moved towards Si. H 3 groups, there will be no lone pair – bond pair repulsion. Hence the structure of (Si. H 3)3 N is planar. However there is no such p-bond formation in (CH 3)3 N and it is pyramidal in shape. IDP in Educational Technology, IIT Bombay 18

In-class Activity Justification for Active Learning Strategy • TPS activity helps students think critically and develop their skills • Increase of students higher order thinking • Peer interaction helps the exchange plenty of knowledge between the students • Overall, there is effective teaching learning in the class room IDP in Educational Technology, IIT Bombay 19