Estimating glucose concentration in solution Introduction The purple




- Slides: 13

Estimating glucose concentration in solution

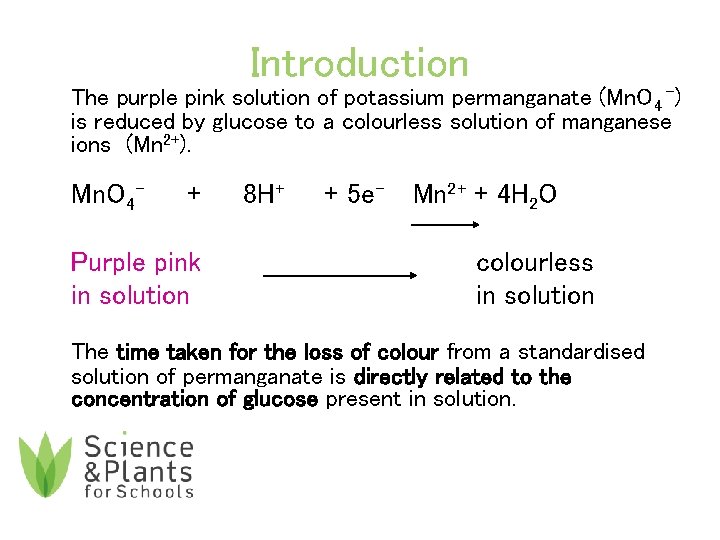
Introduction The purple pink solution of potassium permanganate (Mn. O 4 -) is reduced by glucose to a colourless solution of manganese ions (Mn 2+). Mn. O 4 - + Purple pink in solution 8 H+ + 5 e- Mn 2+ + 4 H 2 O colourless in solution The time taken for the loss of colour from a standardised solution of permanganate is directly related to the concentration of glucose present in solution.

Aims • To determine the effect of increasing glucose concentration on the rate of reaction. • To estimate the concentrations of three glucose solutions of unknown concentration.

Materials required by each group: Eye protection 8 boiling tubes 3 syringes labels/marker pens timer(s) boiling tube rack glass rod Glucose solutions (2, 4, 6, 8 and 10%) 3 glucose solutions of unknown concentration (A, B and C) Sulphuric Acid ( 1 mol dm-3) Potassium permanganate ( 0. 4 g dm-3)

Procedure 1. Label your syringes (‘G’ for glucose, ‘P’ for potassium permanganate and ‘S’ for Sulphuric acid). 2. Label your boiling tubes with the different glucose concentrations to be used. 3. Use the 10 cm 3 syringe to place 10 cm 3 of the correct glucose solution into each boiling tube. Start with the lowest concentration and work up.

Procedure (2) 4. Use the correct syringes to add 5 cm 3 of sulphuric acid to the 2% glucose in the boiling tube. 5. Add 2 cm 3 of potassium permanganate to the same tube and start the clock. 6. Stir with a stirring rod and stop the clock as soon as the pink colour disappears. (Hold boiling tube up against white paper. )

Procedure (3) 7. Record the time taken. 8. Repeat steps 4 – 8 for each glucose solution of known concentration, working from lowest to highest concentration. 9. Repeat steps 3 – 8 for the glucose solutions of unknown concentration (A, B and C).

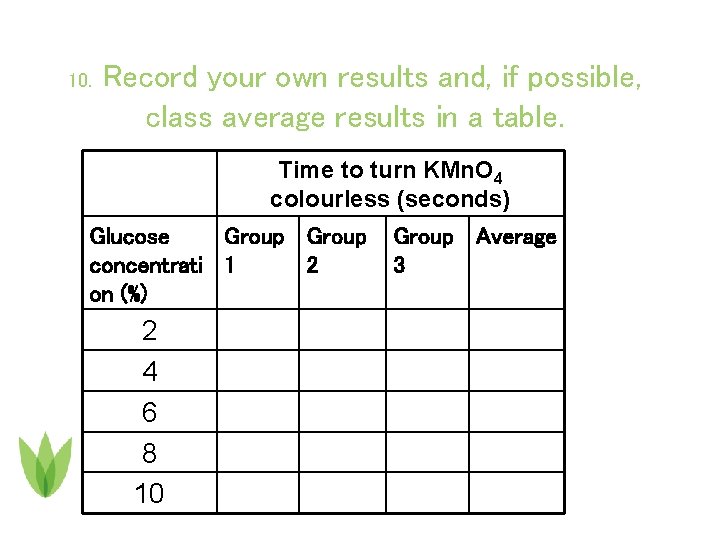
10. Record your own results and, if possible, class average results in a table. Time to turn KMn. O 4 colourless (seconds) Glucose Group concentrati 1 2 on (%) 2 4 6 8 10 Group Average 3

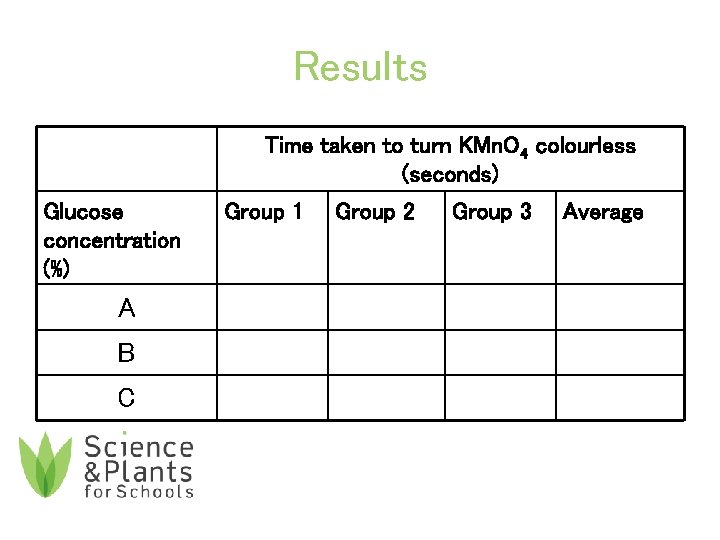
Results Time taken to turn KMn. O 4 colourless (seconds) Glucose concentration (%) A B C Group 1 Group 2 Group 3 Average

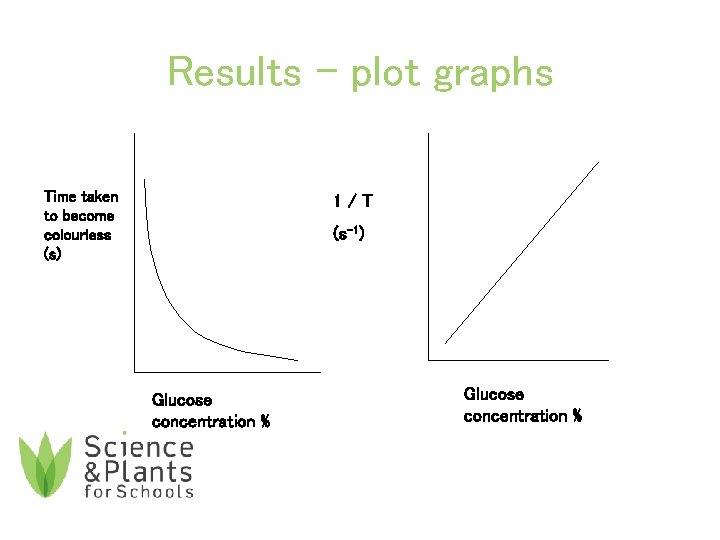
Results 11. Plot a standard curve of your own and the class average results on graph paper. Use the graphs to estimate the concentrations of the unknown solutions. Show your interpolations on the graph. 12. Calculate 1/time using class average results and draw a graph of rate of reaction.

Results – plot graphs Time taken to become colourless (s) 1/T (s-1) Glucose concentration %

Conclusion • Are there any trends in your results? • If so, describe the relationship between glucose concentration and the time taken to turn potassium permanganate colourless/the rate of reaction. • What are the estimated concentrations of your ‘unknown’ solutions?

Evaluation • Ask your teacher for the concentrations of solutions A, B and C and compare the actual concentrations with your estimated concentrations. • Compare your results with the class average results. • Did your experiment work well? • What variables did you control effectively? • What were the sources of error in your experiment? • How could you improve on the design of your experiment and minimise the effects of errors?