Enzyme 3 Factors affecting enzyme activity Lecture NO







- Slides: 23

Enzyme -3. Factors affecting enzyme activity Lecture NO: 1 st MBBS Dr Muhammad Ramzan

Enzyme activity - the definition • Enzyme activity refers to the catalytic ability of an enzyme • to increase the rate of a chemical reaction. • Turnover No: is the maximum number of molecules of substrate that an enzyme : • Can convert to products per catalytic site/ mint. • www. wikipedia. com – www. medical-dictionary. com

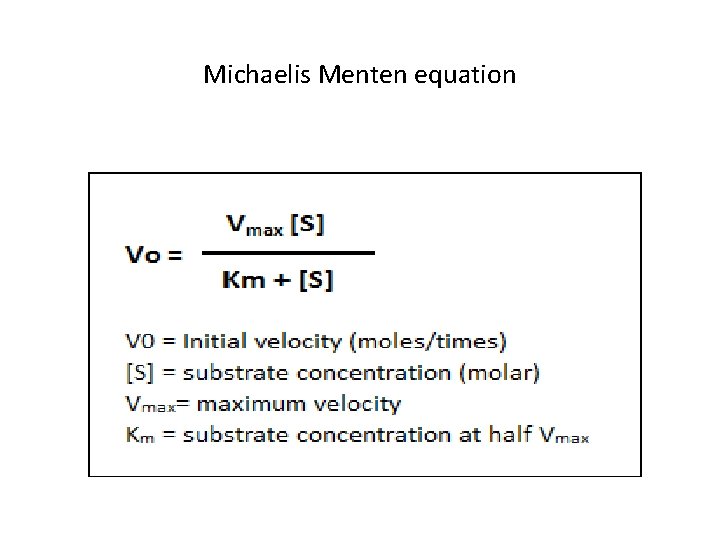
Michaelis-Menten equation • It is a set of mathematical expressions to calculate enzyme activity in terms of speed of reaction from measurable laboratory data.

Michaelis Menten equation

Conclusion of the equation • Characteristic of Km: It reflects the affinity of the E with S • Is equal to the Conc. of S at which reaction velocity is equal to ½ Vmax. Km does not vary with the conc. Of E • It is important in competitive inhibition when ↑ in S reverses the Vmax as inhibitor is diluted • Vmax: is achieved when all the active sites of E are occupied by the S • Vmax: Cannot be achieved in Non completive inhibition as inhibitor binds either to E or ES Complex. • ↑in S has no effect as it cannot bind to active site

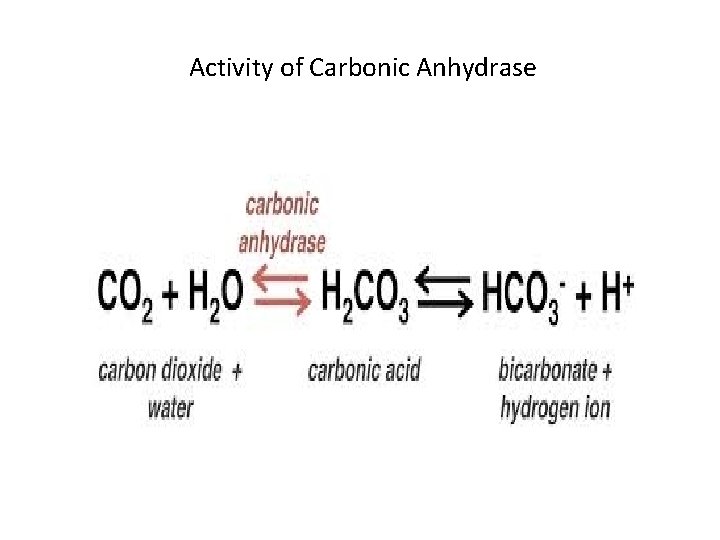
Enzyme assay – measure E activity Carbonic Anhydrase – 36 million. S/mint • Enzymes assay is the laboratory method for measuring the enzyme activity- vital for E kinetics/inhibition • It measures either the disappearance of Substrate or appearance of products overtime • Carbonic Anhydrase is the enzyme having highest Turn Over NO: at 36 million moles per minute. (bicarbonate) • More common NOs: are closer to 1000 moles/minute • www. chem. libretext. org – www. wikipedia. com

Activity of Carbonic Anhydrase

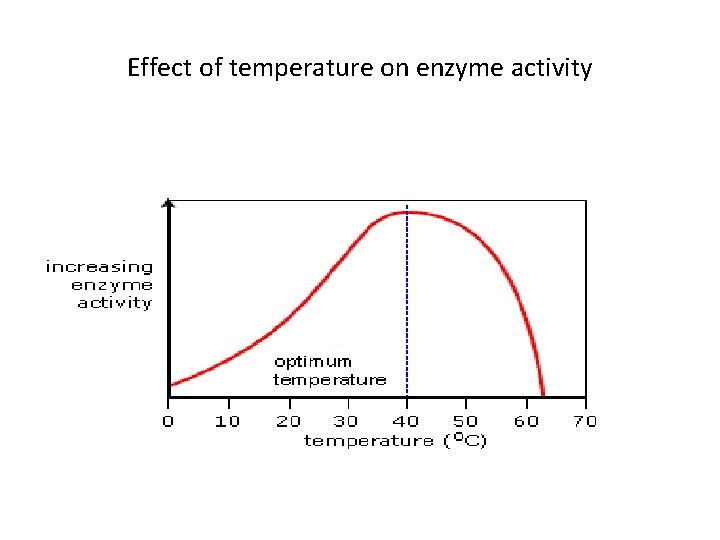
Factors affecting the enzyme activity Temperature and p. H • Although Es speed up chemical reactions, each E works most efficiently under a specific set of conditions. • Because almost all enzymes are proteins, any factor that affects the shape of a protein, may affect E activity • High temperature and hostile p. H are the major factors affecting the activity of the enzymes

Factors affecting enzyme activity the list of 5 • • • These factors are : Temperature p. H of the solution Concentration of substrates and that of enzymes Activators or inhibitor molecules

Enzyme activity and temperature (temp. ) ↑es interaction B/w E and S • Temp. /heat is a sort of energy present in the particles that increases their activity or motion • Temp. ↑ the activity of E and S and rate of reaction so that both Can Interact with each other more frequently • It is more likely that the molecules of S will slot into active site of Enzyme leading to the formation of products

Effect of temperature on enzyme activity

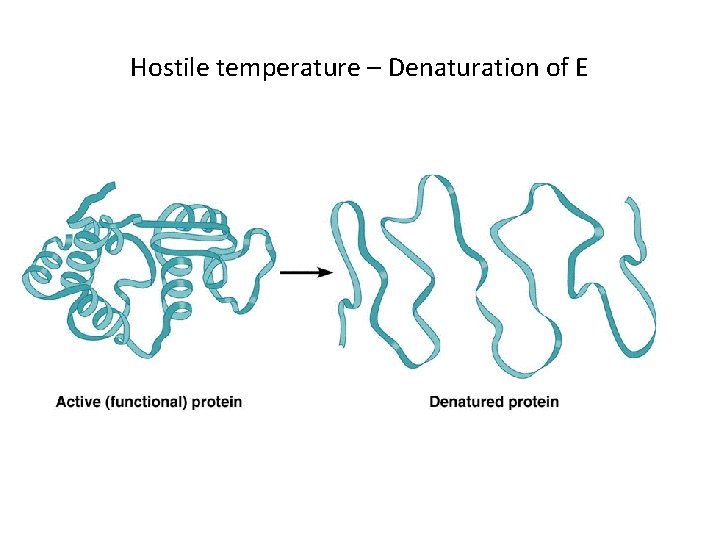
High/hostile tempt. leads to enzyme denaturation • Enzymes are proteins, ↑ of temp. above the optimum level (40 c) causes the protein to lose their : • 3 D structure and folding and: 1 • Breakage of bonds B/w the functional groups of AAs 2 • Change of shape and loss of enzyme function 3 • The optimum temperature for the body enzymes is 37 C°

Hostile temperature – Denaturation of E

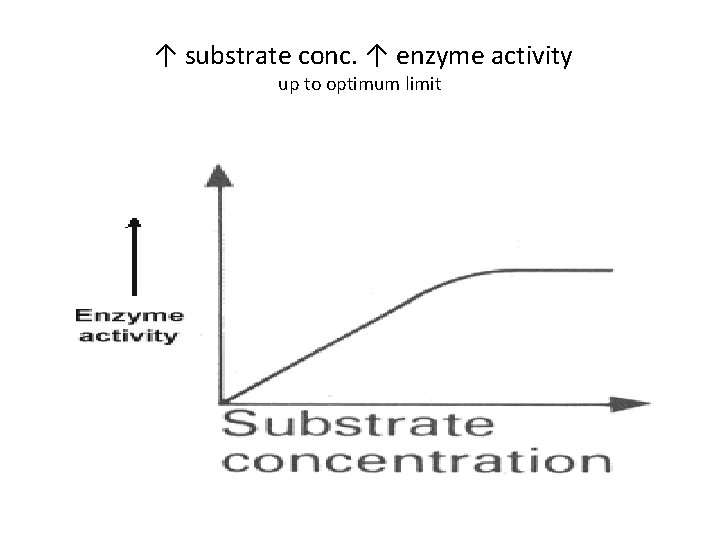
↑ in Substrate conc. ↑es Enzyme activity up to an optimum level • ↑ of substrate ↑the enzyme activity up to an optimum level as more moles of S have the : • Chance to bind with the active site of enzyme • However, excess of S moles, reduces the chances of finding an active site and no further ↑ in enzyme activity

↑ substrate conc. ↑ enzyme activity up to optimum limit

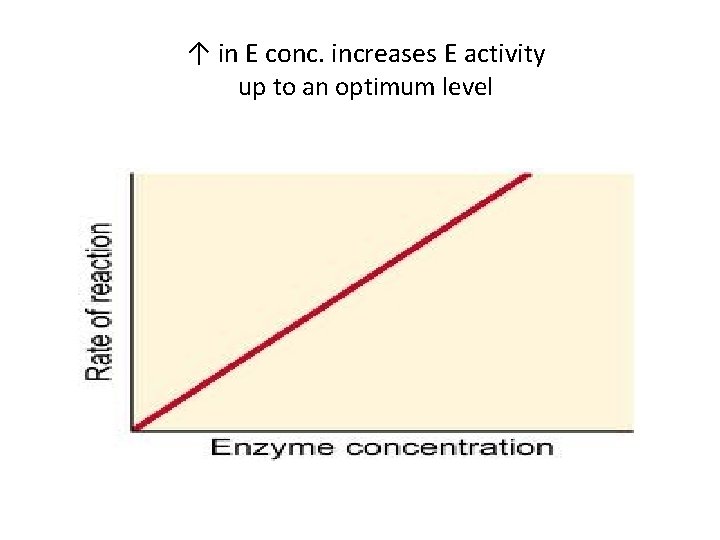
↑ in E conc. ↑ E activity up to an optimum level • This effect is much like that of substrate • ↑ in the enzyme conc. increases the rate of enzyme reaction up to an optimum level • Further ↑ in enzyme conc. has no effect on the reaction activity as no more S molecules are available • However, further ↑ in the substrate molecules, will certainly increase the rate of reaction

↑ in E conc. increases E activity up to an optimum level

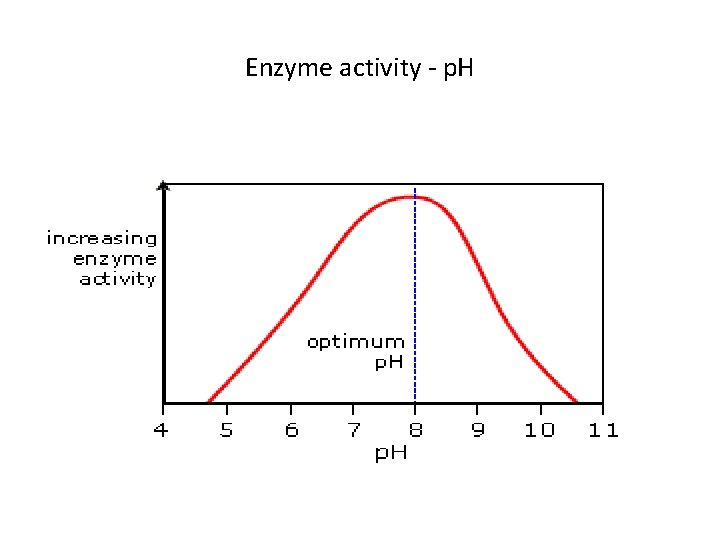
p. H and activity of enzyme up to an optimum level • The p. H level of a solution can also affects enzyme activity. • Many enzymes can only work within a narrow range of p. H • If an enzyme finds itself in a hostile p. H range, it could becomes denatured. • The optimal p. H for many enzymes is 7. 0 -7. 5, but this is not always the case and may be variable

↓ or↑ in p. H – affects E activity Salivary and Gastric enzymes • Enzymes in Gastric secretion like Pepsin and Trypsin work best at an acidic p. H of 1. 5. • while others such as found in the Salivary and Intestinal secretions work best at a more alkaline p. H of 8. 0. • These include Lingual Lipase and Salivary Amylase in saliva • Enzymes in the Intestinal/pancreatic secretions includes: Amylase; Lipase, Protease and carboxy peptidase

Enzyme activity - p. H

Hostile p. H and E activity – the mechanism S non covalent bonds with E • When a substrate slots into the active site of an enzyme • It forms temporary/non covalent bonds with the groups at the active site (Amino acyl groups) • These groups are the functional groups of AA in the protein side chains and have important effects on : • the shape of enzyme

Hostile p. H changes the shape of enzyme • If functional groups of AAs side chain are in different order, the S would not bind temporary to the E as : • the shape of enzyme will be different • This is what happens when the p. H changes: • increases or decreases

Enzyme activity – the inhibition • Enzyme inhibition is the process when a chemical substance binds to the active site of an enzyme and: • Inhibits activity so that active site is no more available to the substrates • Inhibitor can bind to active site, Allosteric site or ESC • Such substances are called enzyme inhibitors • These inhibitors can cause irreversible or reversible inhibition • Reversible inhibition can be competitive and non competitive