Entropy Second Law of Thermodynamics PHYS116 A02 4182013

- Slides: 18

Entropy, Second Law of Thermodynamics PHYS-116 A-02 4/18/2013 Lecture 24 Momchil Velkovsky

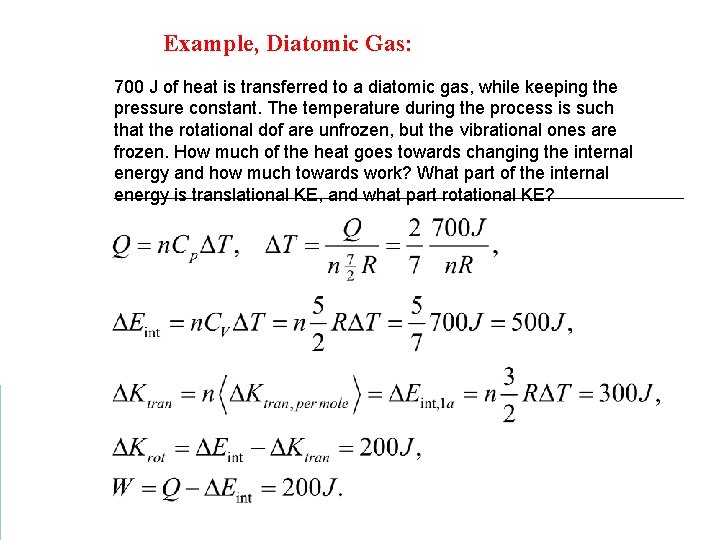
Example, Diatomic Gas: 700 J of heat is transferred to a diatomic gas, while keeping the pressure constant. The temperature during the process is such that the rotational dof are unfrozen, but the vibrational ones are frozen. How much of the heat goes towards changing the internal energy and how much towards work? What part of the internal energy is translational KE, and what part rotational KE?

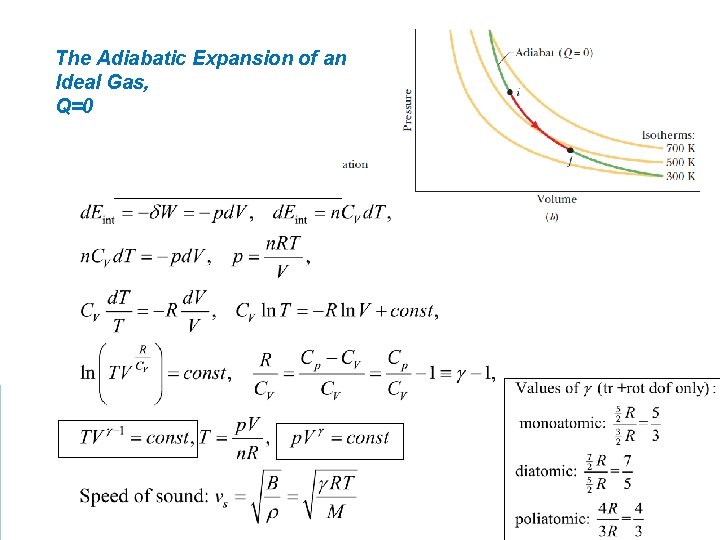
The Adiabatic Expansion of an Ideal Gas, Q=0

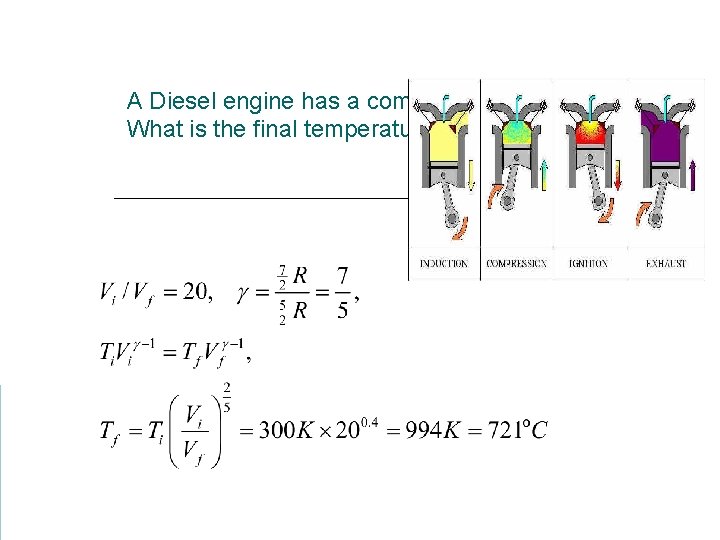
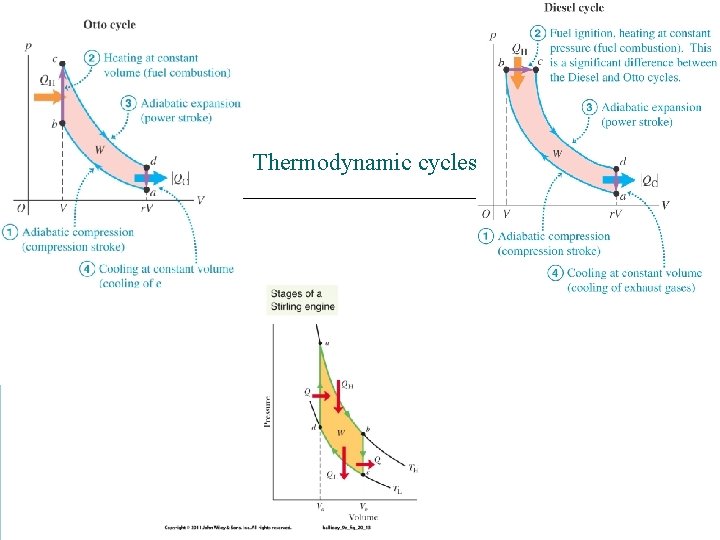
A Diesel engine has a compression ratio of 20: 1 What is the final temperature in the cylinder?

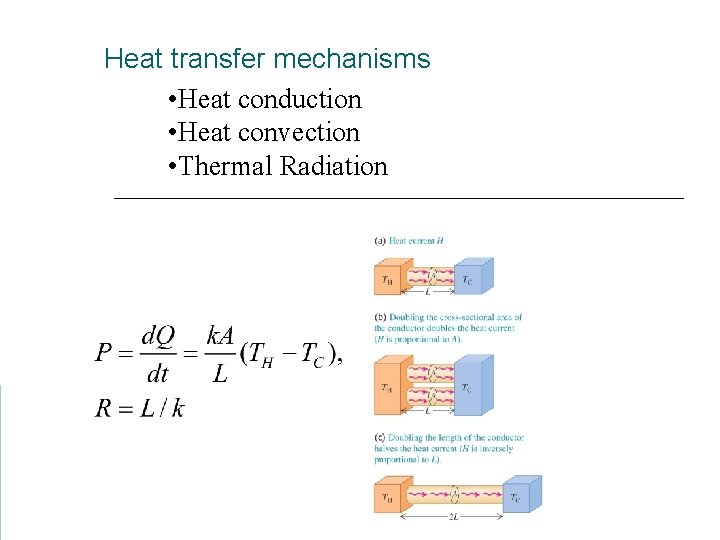
Heat transfer mechanisms • Heat conduction • Heat convection • Thermal Radiation

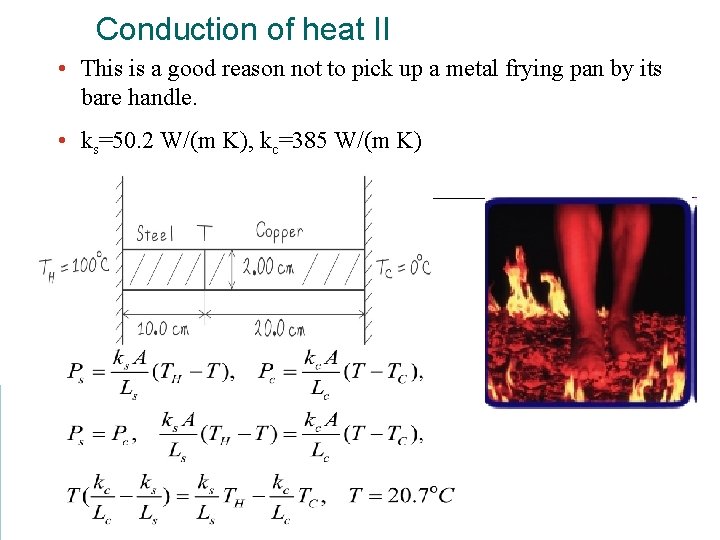
Conduction of heat II • This is a good reason not to pick up a metal frying pan by its bare handle. • ks=50. 2 W/(m K), kc=385 W/(m K)

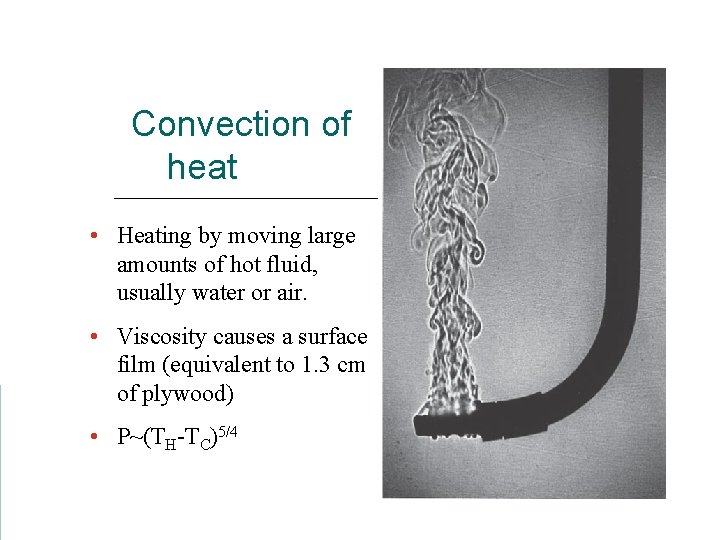
Convection of heat • Heating by moving large amounts of hot fluid, usually water or air. • Viscosity causes a surface film (equivalent to 1. 3 cm of plywood) • P~(TH-TC)5/4

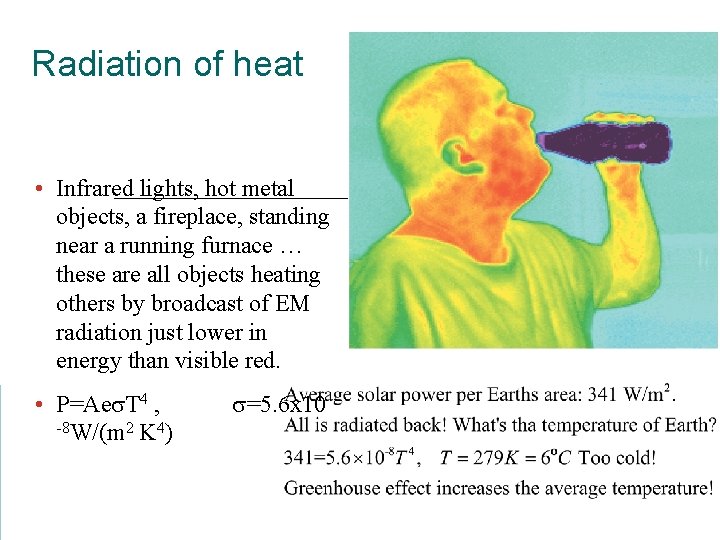
Radiation of heat • Infrared lights, hot metal objects, a fireplace, standing near a running furnace … these are all objects heating others by broadcast of EM radiation just lower in energy than visible red. • P=Aes. T 4 , -8 W/(m 2 K 4) s=5. 6 x 10

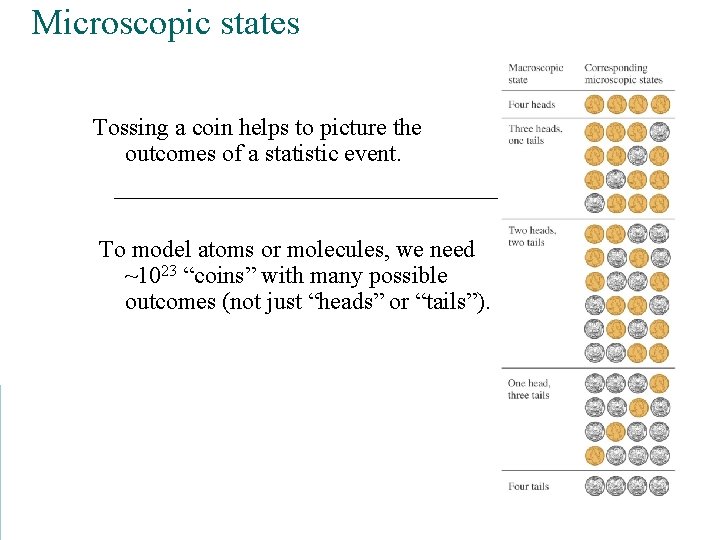
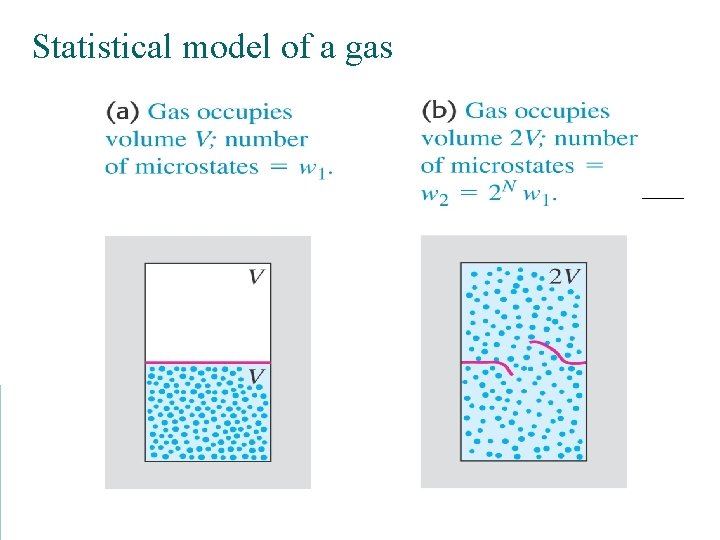
Microscopic states Tossing a coin helps to picture the outcomes of a statistic event. To model atoms or molecules, we need ~1023 “coins” with many possible outcomes (not just “heads” or “tails”).

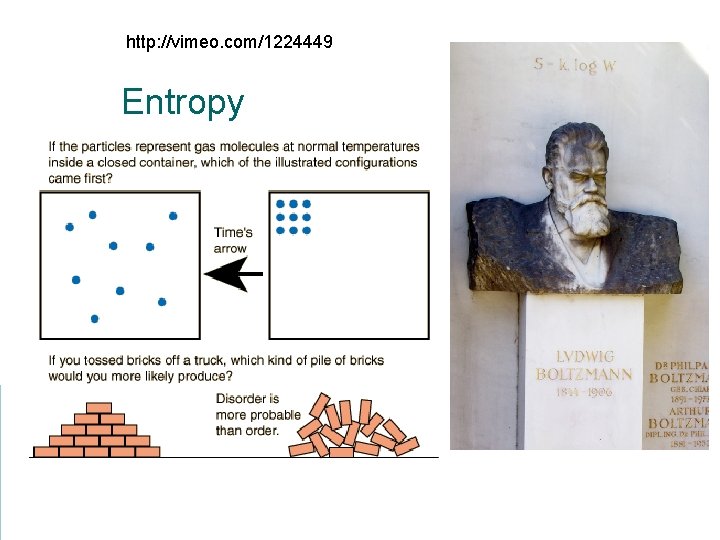
http: //vimeo. com/1224449 Entropy

Statistical model of a gas

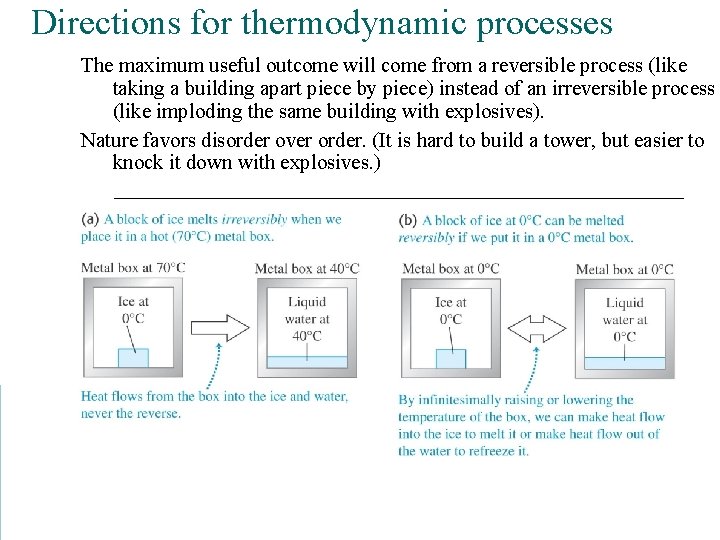
Directions for thermodynamic processes The maximum useful outcome will come from a reversible process (like taking a building apart piece by piece) instead of an irreversible process (like imploding the same building with explosives). Nature favors disorder over order. (It is hard to build a tower, but easier to knock it down with explosives. )

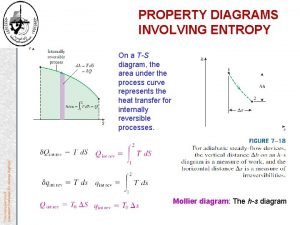
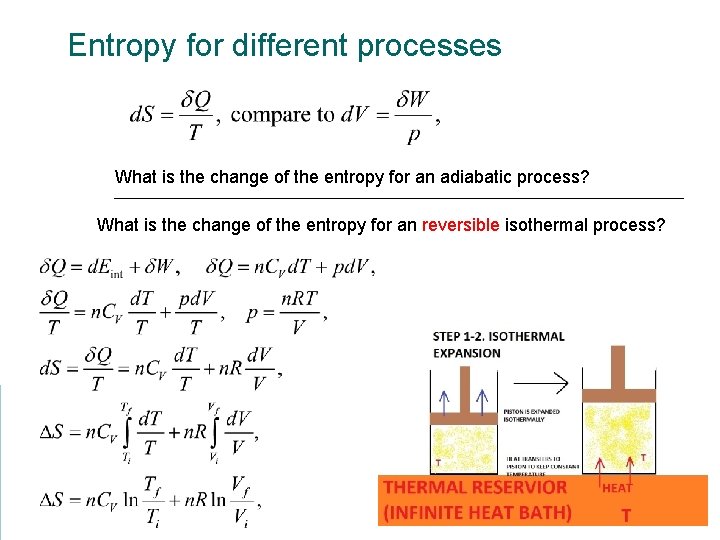
Entropy for different processes What is the change of the entropy for an adiabatic process? What is the change of the entropy for an reversible isothermal process?

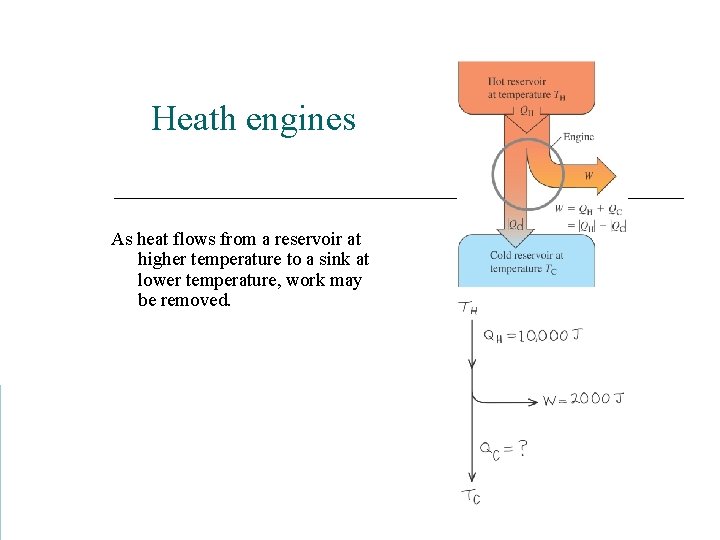
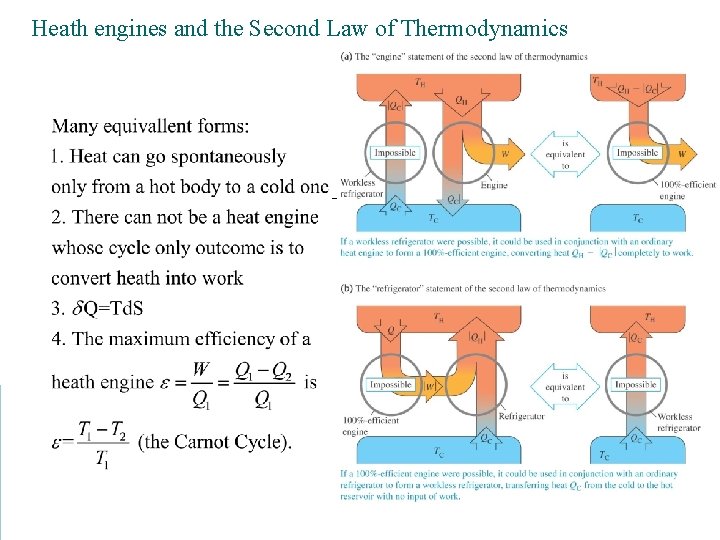
Heath engines As heat flows from a reservoir at higher temperature to a sink at lower temperature, work may be removed.

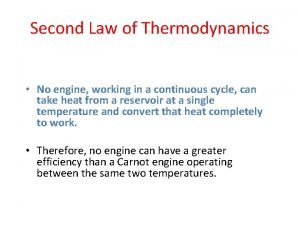
Heath engines and the Second Law of Thermodynamics

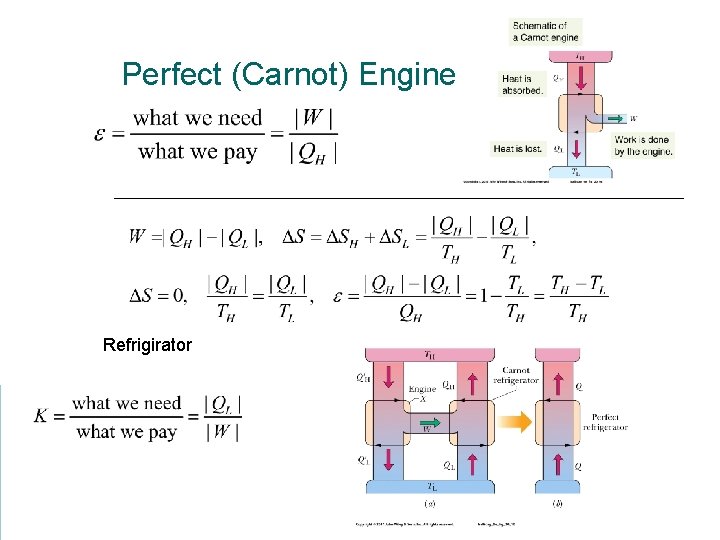
Perfect (Carnot) Engine Refrigirator

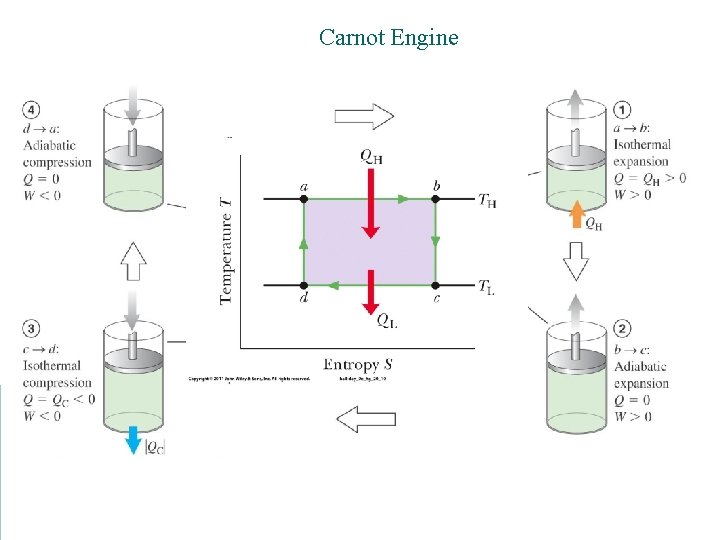
Carnot Engine

Thermodynamic cycles
 Change in entropy formula
Change in entropy formula State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Second law of thermodynamics definition
Second law of thermodynamics definition Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Thermodynamics
Thermodynamics Entropy in thermodynamics
Entropy in thermodynamics What is entropy in thermodynamics
What is entropy in thermodynamics Entropy of ideal gas
Entropy of ideal gas Law of entropy
Law of entropy 186 282 miles per second into meters per second
186 282 miles per second into meters per second First law of thermodynamics in open system
First law of thermodynamics in open system Zeroth law example
Zeroth law example Newtons third law of thermodynamics
Newtons third law of thermodynamics Thermodynamics laws
Thermodynamics laws