Entropy Changes in Chemical Reactions Tro Chapter 18
- Slides: 13

Entropy Changes in Chemical Reactions Tro Chapter 18 – Free Energy and Thermodynamics 18. 7 Entropy Changes in Chemical Reactions: Calculating DSorxn

The Third Law of Thermodynamics: Absolute Entropy • The absolute entropy of a substance is the amount of energy it has due to dispersion of energy through its particles. • The third law states that the entropy for a perfect crystal at 0 K is zero. • Absolute entropy = 0 J/mol ∙ K • A substance that isn’t a perfect crystal at absolute zero has some energy from entropy. • The absolute entropy of substances is always positive.

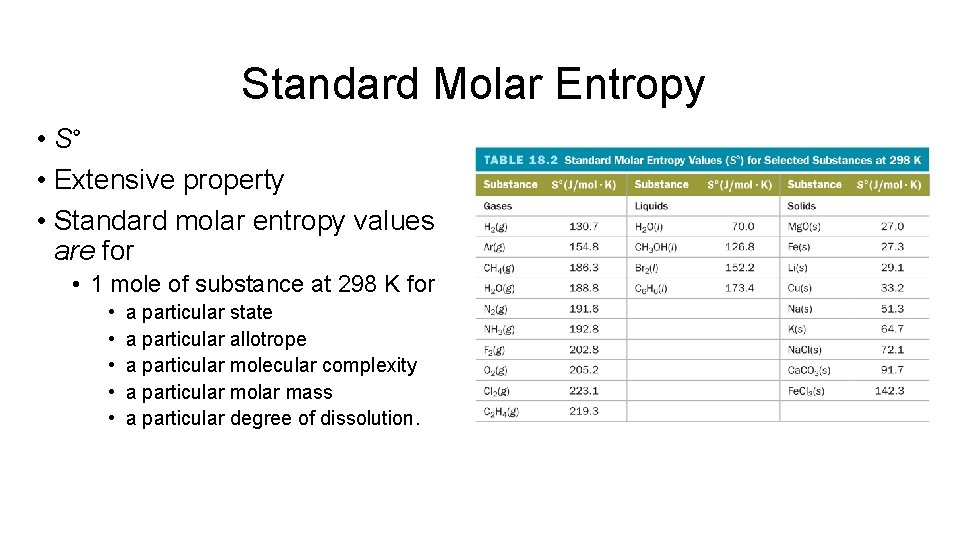
Standard Molar Entropy • S° • Extensive property • Standard molar entropy values are for • 1 mole of substance at 298 K for • • • a particular state a particular allotrope a particular molecular complexity a particular molar mass a particular degree of dissolution.

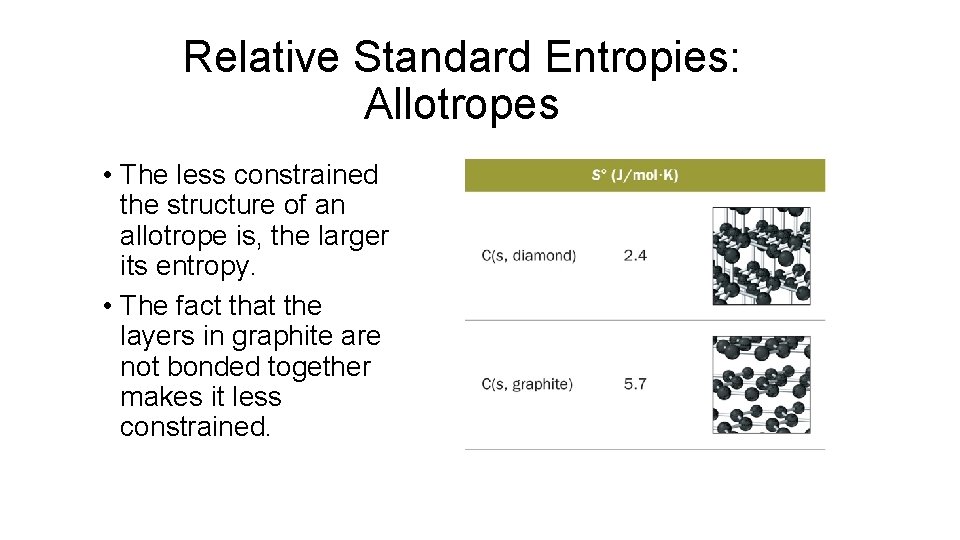
Relative Standard Entropies: Allotropes • The less constrained the structure of an allotrope is, the larger its entropy. • The fact that the layers in graphite are not bonded together makes it less constrained.

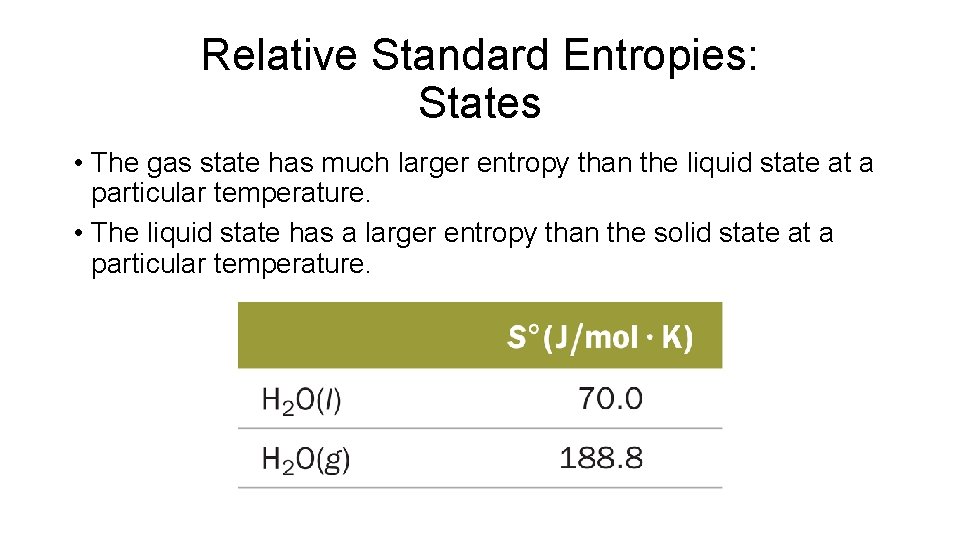
Relative Standard Entropies: States • The gas state has much larger entropy than the liquid state at a particular temperature. • The liquid state has a larger entropy than the solid state at a particular temperature.

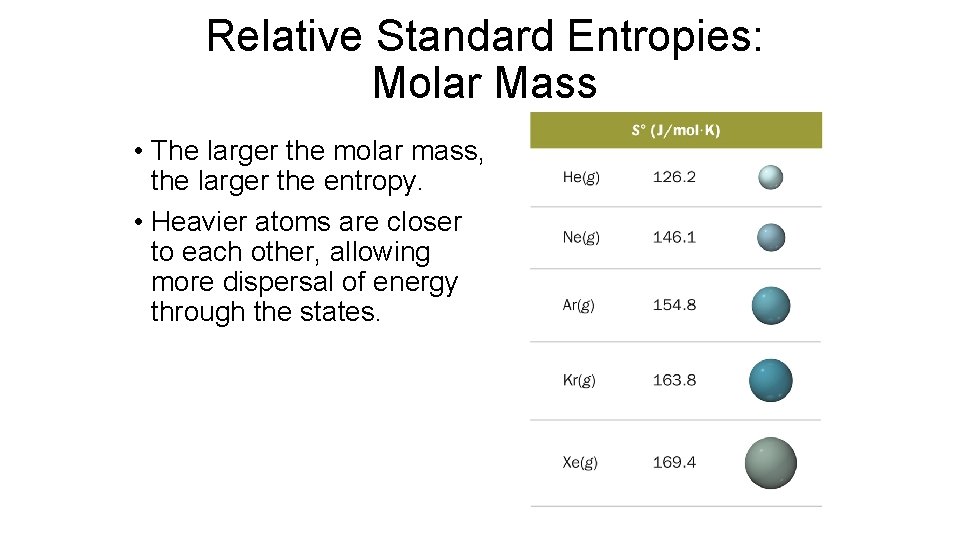
Relative Standard Entropies: Molar Mass • The larger the molar mass, the larger the entropy. • Heavier atoms are closer to each other, allowing more dispersal of energy through the states.

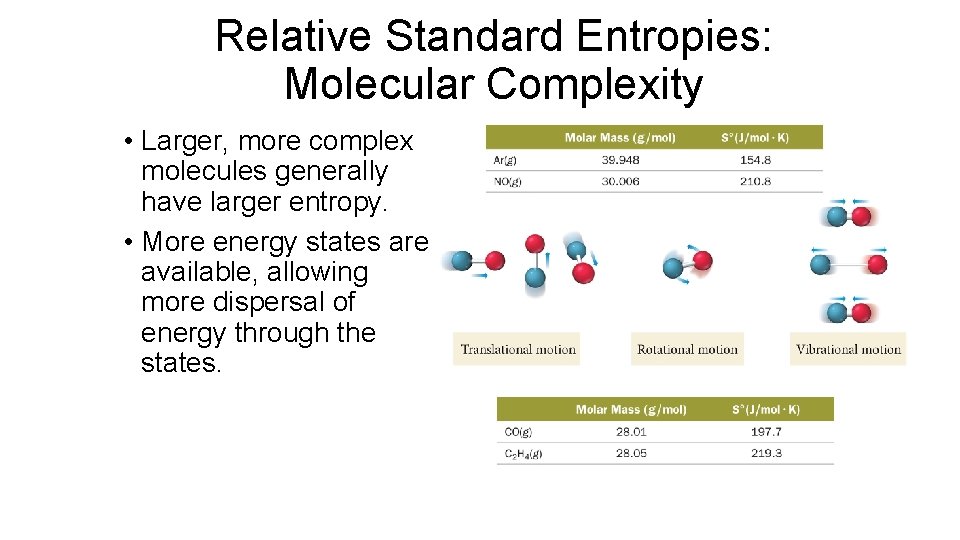
Relative Standard Entropies: Molecular Complexity • Larger, more complex molecules generally have larger entropy. • More energy states are available, allowing more dispersal of energy through the states.

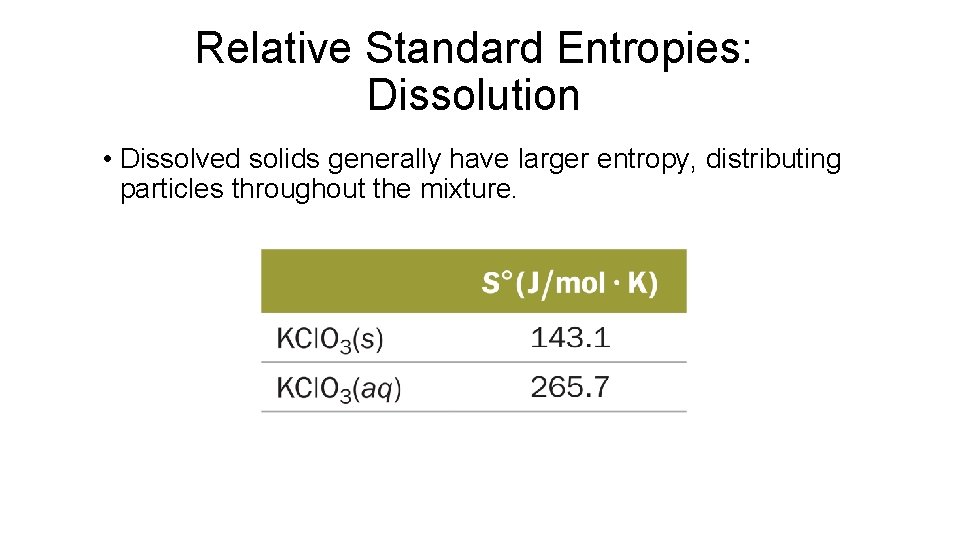
Relative Standard Entropies: Dissolution • Dissolved solids generally have larger entropy, distributing particles throughout the mixture.

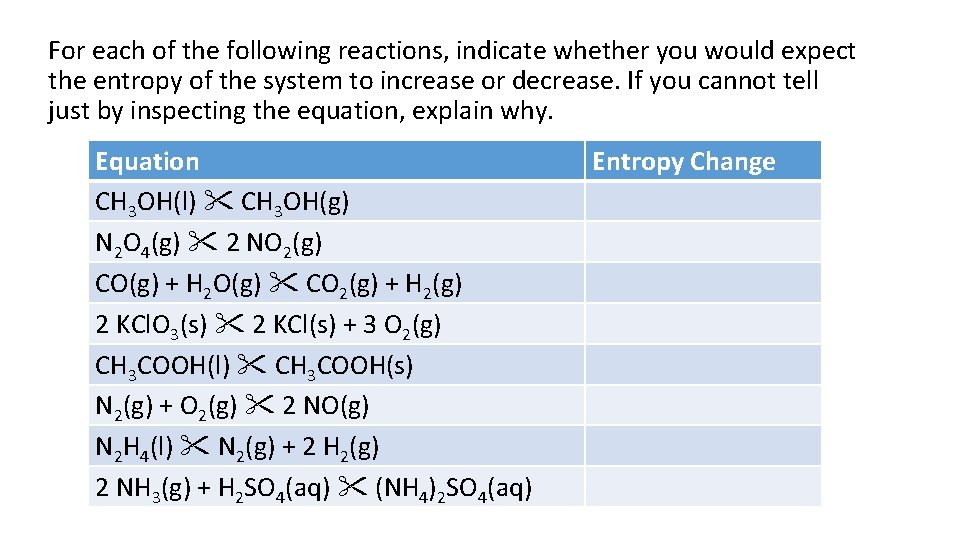
For each of the following reactions, indicate whether you would expect the entropy of the system to increase or decrease. If you cannot tell just by inspecting the equation, explain why. Equation CH 3 OH(l) CH 3 OH(g) N 2 O 4(g) 2 NO 2(g) CO(g) + H 2 O(g) CO 2(g) + H 2(g) 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) CH 3 COOH(l) CH 3 COOH(s) N 2(g) + O 2(g) 2 NO(g) N 2 H 4(l) N 2(g) + 2 H 2(g) 2 NH 3(g) + H 2 SO 4(aq) (NH 4)2 SO 4(aq) Entropy Change

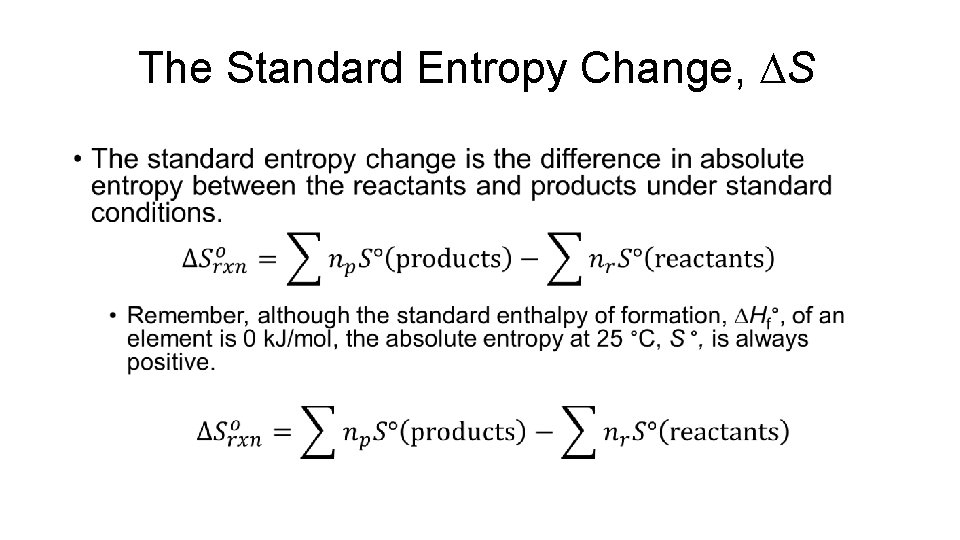
The Standard Entropy Change, DS •

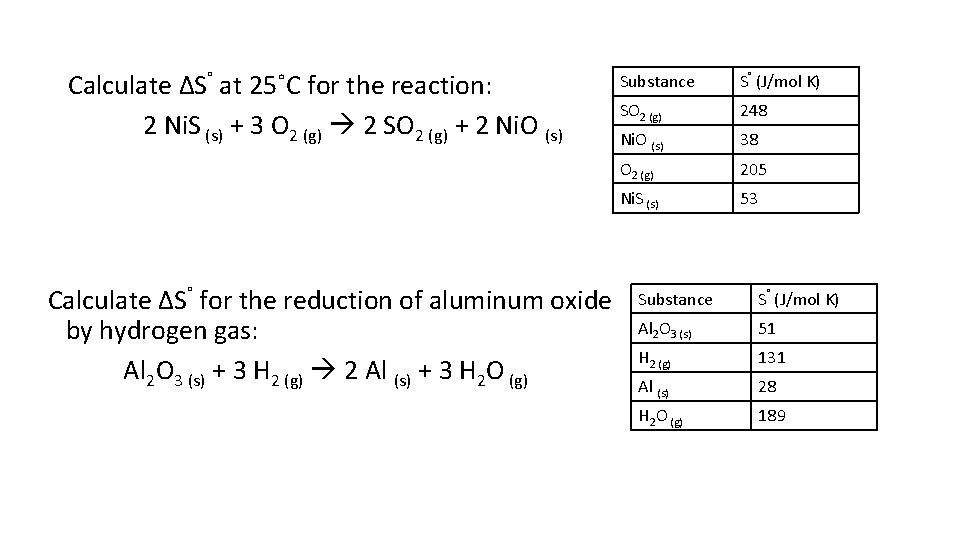
Calculate ΔS˚ at 25˚C for the reaction: 2 Ni. S (s) + 3 O 2 (g) 2 SO 2 (g) + 2 Ni. O (s) Calculate ΔS˚ for the reduction of aluminum oxide by hydrogen gas: Al 2 O 3 (s) + 3 H 2 (g) 2 Al (s) + 3 H 2 O (g) Substance S˚ (J/mol K) SO 2 (g) 248 Ni. O (s) 38 O 2 (g) 205 Ni. S (s) 53 Substance S˚ (J/mol K) Al 2 O 3 (s) 51 H 2 (g) 131 Al (s) 28 H 2 O (g) 189

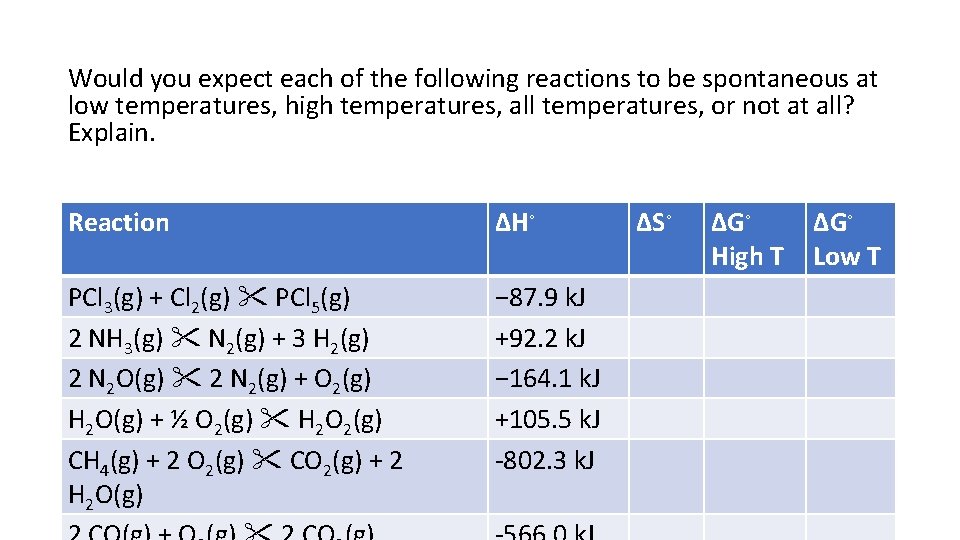
Would you expect each of the following reactions to be spontaneous at low temperatures, high temperatures, all temperatures, or not at all? Explain. Reaction ΔH◦ PCl 3(g) + Cl 2(g) PCl 5(g) 2 NH 3(g) N 2(g) + 3 H 2(g) 2 N 2 O(g) 2 N 2(g) + O 2(g) H 2 O(g) + ½ O 2(g) H 2 O 2(g) CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) − 87. 9 k. J +92. 2 k. J − 164. 1 k. J +105. 5 k. J -802. 3 k. J ΔS◦ ΔG◦ High T ΔG◦ Low T

The following reaction is spontaneous under standard state conditions at room temperature. CO(g) + Cl 2(g) COCl 2(g) To make it a nonspontaneous reaction, would you raise or lower the temperature? Explain.