Elements Compounds and Mixtures Elements Made Can up



- Slides: 12

Elements, Compounds, and Mixtures

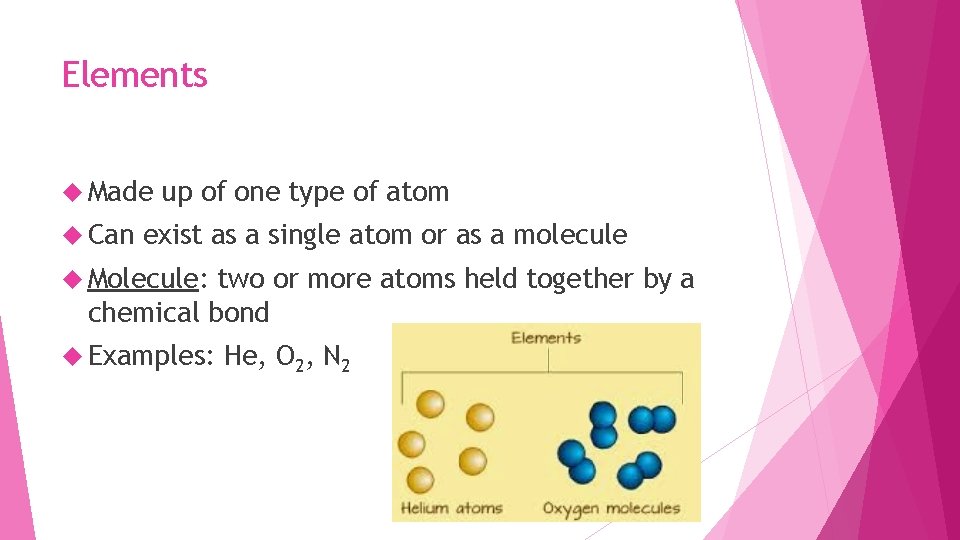
Elements Made Can up of one type of atom exist as a single atom or as a molecule Molecule: two or more atoms held together by a chemical bond Examples: He, O 2, N 2

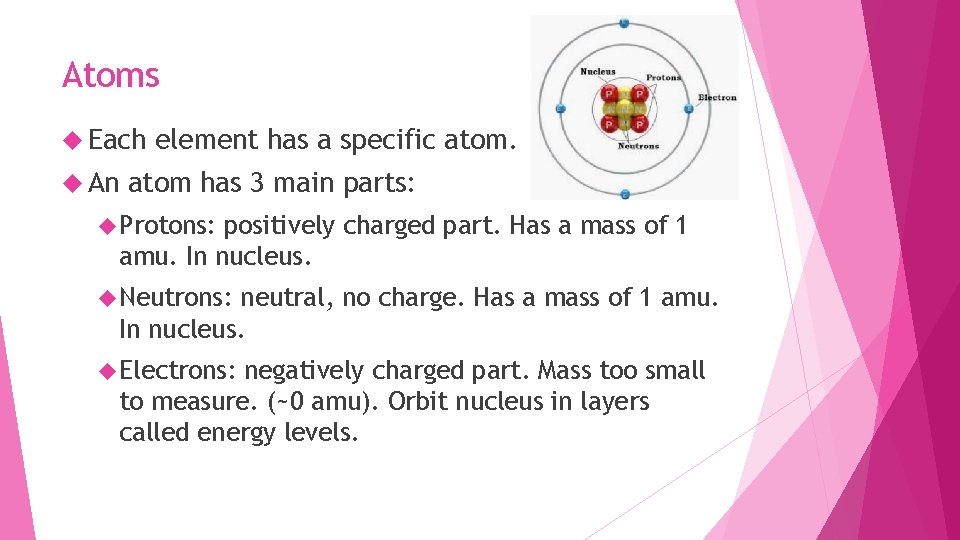
Atoms Each An element has a specific atom has 3 main parts: Protons: positively charged part. Has a mass of 1 amu. In nucleus. Neutrons: neutral, no charge. Has a mass of 1 amu. In nucleus. Electrons: negatively charged part. Mass too small to measure. (~0 amu). Orbit nucleus in layers called energy levels.

Compounds When atoms of different elements join together by chemical bonds Always exist as molecules (in same ratio) Properties are different from the component elements Examples: H 2 O, CO 2, C 6 H 12 O 6

Mixtures When more than one substance are mixed together. Can be made from elements &/or compounds The substances do not join chemically and they keep their own properties. Substances Examples: can be separated out of a mixture air, sand & water, bag of skittles

Periodic Table A chart organizing information about all known elements Periods- the rows (across) of the periodic table Corresponds Groups- to electron energy level the columns (up/down) of the periodic table Atoms in the same group have the same set up of their outer (valence) electrons Elements in the same group behave similarly

Atomic Arrangement- nucleus Protons and neutrons are located in the nucleus # protons = atomic number (this defines the element) # neutrons = atomic mass – atomic number Round atomic mass to a whole number. The number is a decimal because it is an average Isotope: atoms of the same element with different number of neutrons.

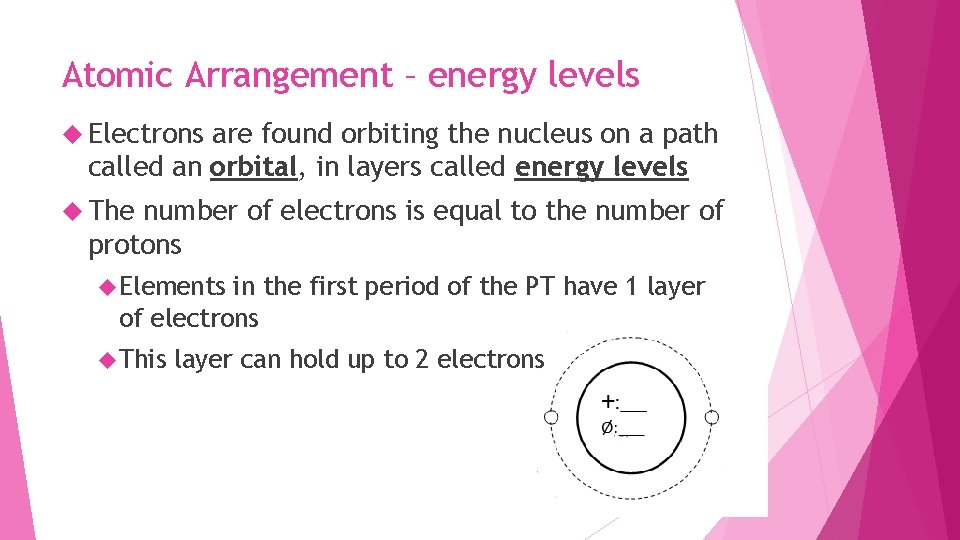
Atomic Arrangement – energy levels Electrons are found orbiting the nucleus on a path called an orbital, in layers called energy levels The number of electrons is equal to the number of protons Elements in the first period of the PT have 1 layer of electrons This layer can hold up to 2 electrons

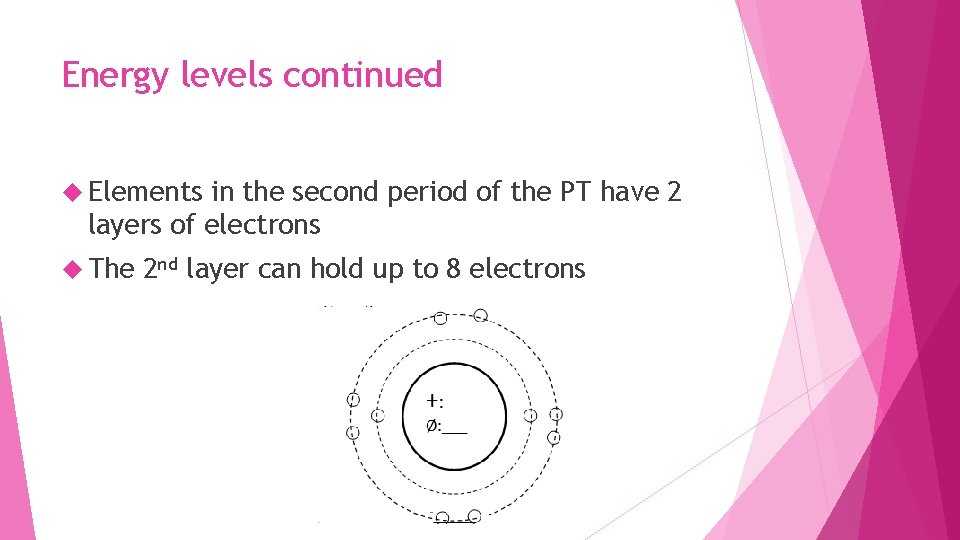
Energy levels continued Elements in the second period of the PT have 2 layers of electrons The 2 nd layer can hold up to 8 electrons

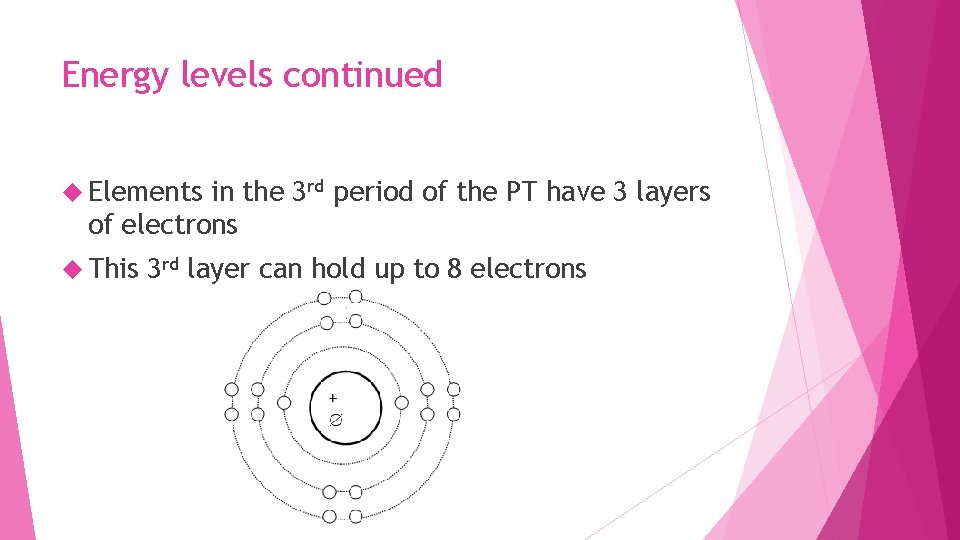
Energy levels continued Elements in the 3 rd period of the PT have 3 layers of electrons This 3 rd layer can hold up to 8 electrons

Valence Electrons in the outer layer are called valence electrons Atoms want to gain, lose, or share electrons in order to have a full outer layer When an atom gains or loses an electron, the atom is now charged and is called an ion Anion: an atom that has gained an electron and has a negative charge (-ide) Cation: an atom that has lost an electron and has a positive charge

Bonding Atoms form bonds to complete their outer layer of electrons Ionic bond: when oppositely charged ions attract, forming an ionic compound. Covalent bond: when atoms share one or more pairs of electrons (give one, get one) This happens when both atoms have almost full outer shells.