Dual Antiplatelet Therapy After DES When is it






- Slides: 23

Dual Antiplatelet Therapy After DES: When is it Safe to Stop? Laura Mauri, MD, MSc Brigham and Women’s Hospital Harvard Medical School

Laura Mauri, MD § Consulting Fees: § Cordis Corporation § Abbott § Medtronic §Research support to Harvard Clinical Research Institute from: §Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Inc. , Bristol-Myers Squibb/Sanofi-Aventis Pharmaceuticals Partnership, Eli Lilly and Company and Daiichi Sankyo Company Limited

Dual Antiplatelet Therapy After DES: When is it Safe to Stop? • We don’t know! • Broad variation in practice reflects this uncertainty • Most data on duration are observational • A few small randomized studies have been released, several larger studies underway

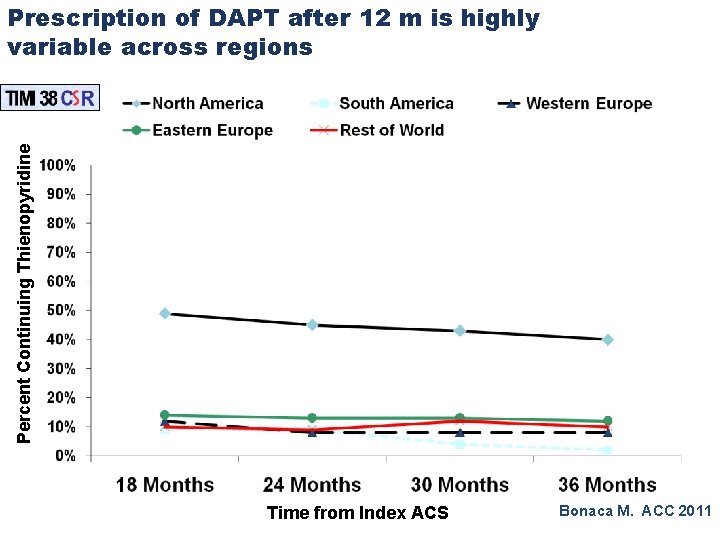
Percent Continuing Thienopyridine Prescription of DAPT after 12 m is highly variable across regions Time from Index ACS Bonaca M. ACC 2011

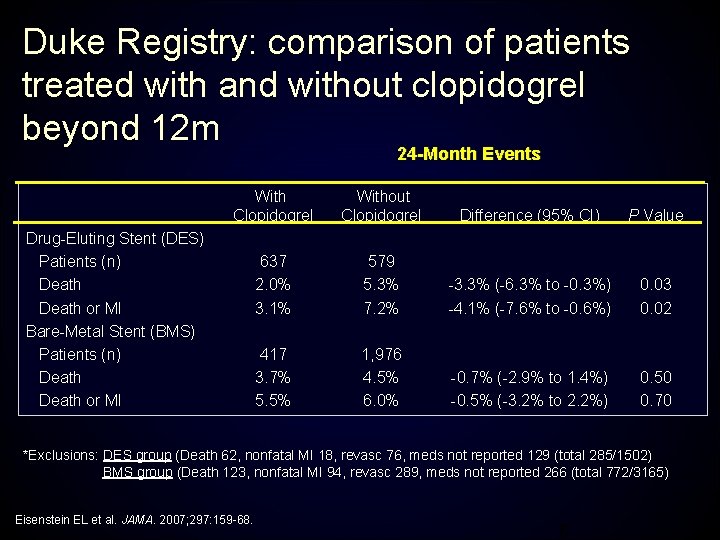
Duke Registry: comparison of patients treated with and without clopidogrel beyond 12 m 24 -Month Events With Clopidogrel Without Clopidogrel Difference (95% CI) P Value 637 2. 0% 3. 1% 579 5. 3% 7. 2% -3. 3% (-6. 3% to -0. 3%) -4. 1% (-7. 6% to -0. 6%) 0. 03 0. 02 417 3. 7% 5. 5% 1, 976 4. 5% 6. 0% -0. 7% (-2. 9% to 1. 4%) -0. 5% (-3. 2% to 2. 2%) 0. 50 0. 70 Drug-Eluting Stent (DES) Patients (n) Death or MI Bare-Metal Stent (BMS) Patients (n) Death or MI *Exclusions: DES group (Death 62, nonfatal MI 18, revasc 76, meds not reported 129 (total 285/1502) BMS group (Death 123, nonfatal MI 94, revasc 289, meds not reported 266 (total 772/3165) Eisenstein EL et al. JAMA. 2007; 297: 159 -68. 6

Comparing Durations of Antiplatelet Therapy • Goal is to define the balance of ischemic vs bleeding risk • Stent thrombosis is a rare event, that requires data review and adjudication, but has profound clinical significance, not available in most registries • The presence of a small confounder could reverse the findings of an observational study on this rare event • Large, and inclusive, randomized trial results are needed

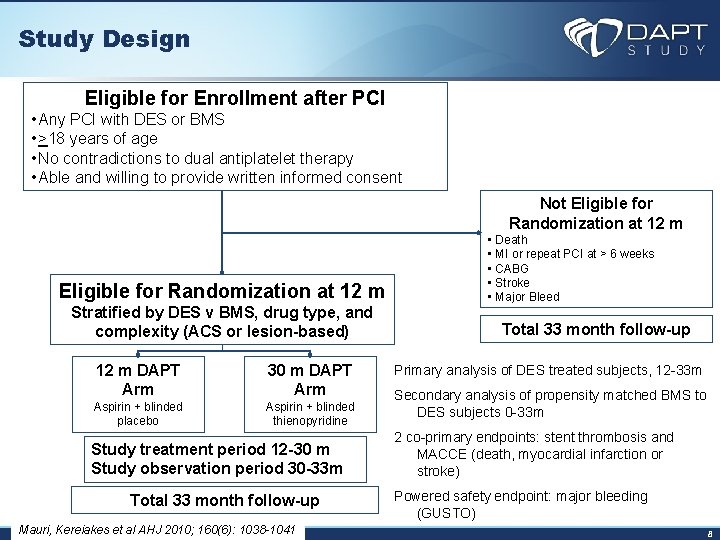
Study Design Eligible for Enrollment after PCI • Any PCI with DES or BMS • >18 years of age • No contradictions to dual antiplatelet therapy • Able and willing to provide written informed consent Not Eligible for Randomization at 12 m Stratified by DES v BMS, drug type, and complexity (ACS or lesion-based) 12 m DAPT Arm 30 m DAPT Arm Aspirin + blinded placebo Aspirin + blinded thienopyridine Study treatment period 12 -30 m Study observation period 30 -33 m Total 33 month follow-up Mauri, Kereiakes et al AHJ 2010; 160(6): 1038 -1041 • Death • MI or repeat PCI at > 6 weeks • CABG • Stroke • Major Bleed Total 33 month follow-up Primary analysis of DES treated subjects, 12 -33 m Secondary analysis of propensity matched BMS to DES subjects 0 -33 m 2 co-primary endpoints: stent thrombosis and MACCE (death, myocardial infarction or stroke) Powered safety endpoint: major bleeding (GUSTO) 8

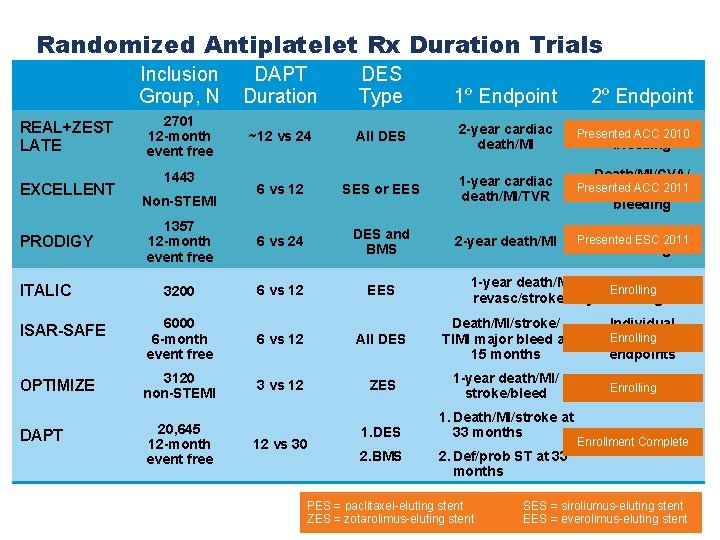
Randomized Antiplatelet Rx Duration Trials Inclusion Group, N DAPT Duration DES Type 2701 12 -month event free ~12 vs 24 All DES 2 -year cardiac death/MI ARC ST, 2010 Presented ACC 6 vs 12 SES or EES 1 -year cardiac death/MI/TVR Death/MI/CVA/ Presented ACC 2011 ST/major bleeding 1357 12 -month event free 6 vs 24 DES and BMS 2 -year death/MI ARC ST, Presented ESC 2011 3200 6 vs 12 EES ISAR-SAFE 6000 6 -month event free 6 vs 12 All DES Death/MI/stroke/ TIMI major bleed at 15 months Individual Enrolling component endpoints OPTIMIZE 3120 non-STEMI 3 vs 12 ZES 1 -year death/MI/ stroke/bleed ARC ST Enrolling DAPT 20, 645 12 -month event free REAL+ZEST LATE EXCELLENT PRODIGY ITALIC 1443 Non-STEMI 12 vs 30 1. DES 2. BMS 1º Endpoint 2º Endpoint bleeding 1 -year death/MI/repeat urgent Enrolling revasc/stroke/majorbleeding 1. Death/MI/stroke at 33 months 2. Def/prob ST at 33 months PES = paclitaxel-eluting stent ZES = zotarolimus-eluting stent Enrollment Complete Major bleeding SES = siroliumus-eluting stent EES = everolimus-eluting stent

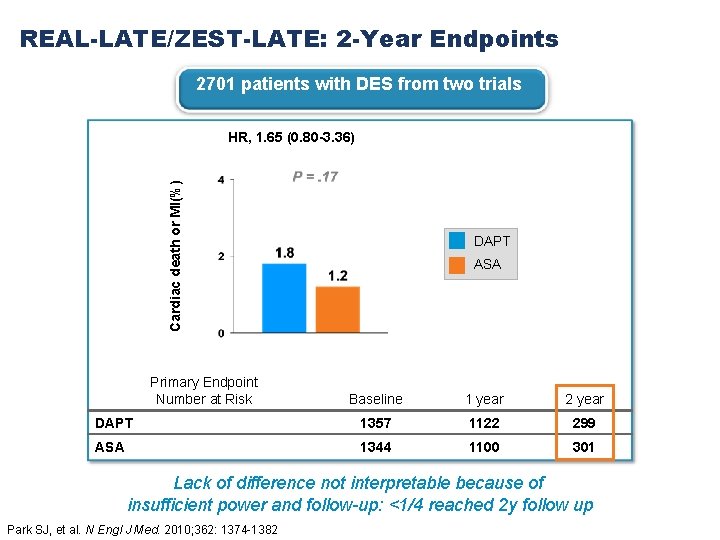
REAL-LATE/ZEST-LATE: 2 -Year Endpoints 2701 patients with DES from two trials Cardiac death or MI(%) HR, 1. 65 (0. 80 -3. 36) Primary Endpoint Number at Risk DAPT ASA Baseline 1 year 2 year DAPT 1357 1122 299 ASA 1344 1100 301 Lack of difference not interpretable because of insufficient power and follow-up: <1/4 reached 2 y follow up Park SJ, et al. N Engl J Med. 2010; 362: 1374 -1382

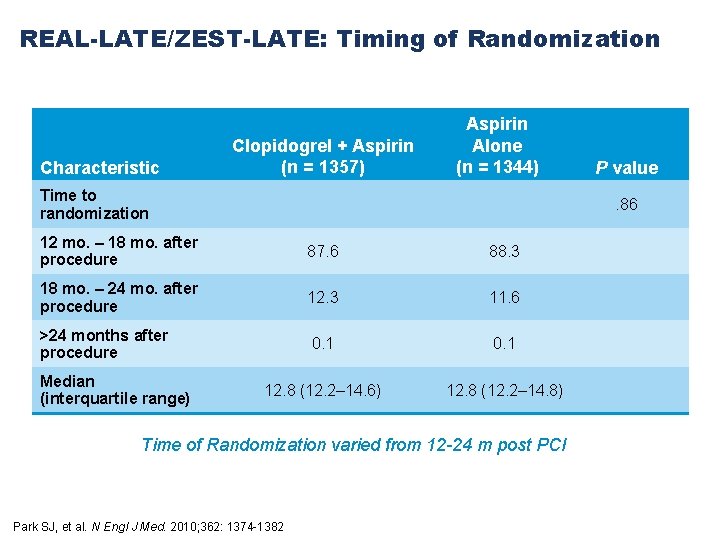
REAL-LATE/ZEST-LATE: Timing of Randomization Characteristic Clopidogrel + Aspirin (n = 1357) Aspirin Alone (n = 1344) Time to randomization . 86 12 mo. – 18 mo. after procedure 87. 6 88. 3 18 mo. – 24 mo. after procedure 12. 3 11. 6 >24 months after procedure 0. 1 12. 8 (12. 2– 14. 6) 12. 8 (12. 2– 14. 8) Median (interquartile range) P value Time of Randomization varied from 12 -24 m post PCI Park SJ, et al. N Engl J Med. 2010; 362: 1374 -1382

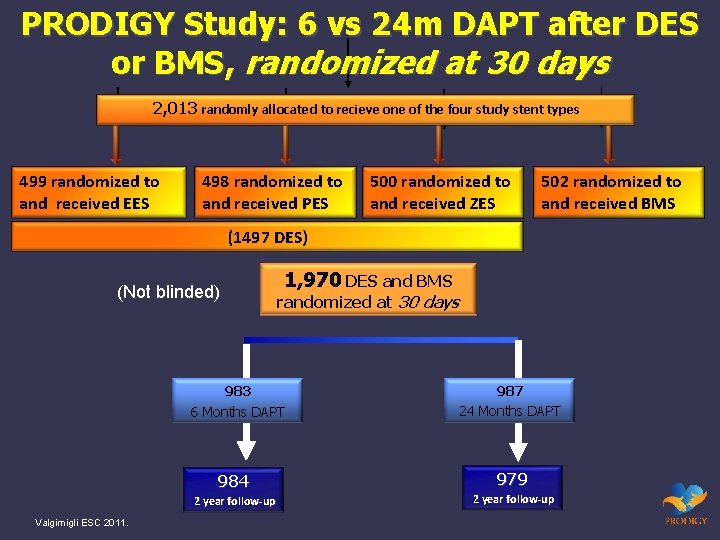
PRODIGY Study: 6 vs 24 m DAPT after DES or BMS, randomized at 30 days 2, 013 randomly allocated to recieve one of the four study stent types 499 randomized to and received EES 498 randomized to and received PES 500 randomized to and received ZES 502 randomized to and received BMS (1497 DES) (Not blinded) randomized at 30 days 983 6 Months DAPT 987 24 Months DAPT 984 979 2 year follow-up Valgimigli ESC 2011. 1, 970 DES and BMS 2 year follow-up

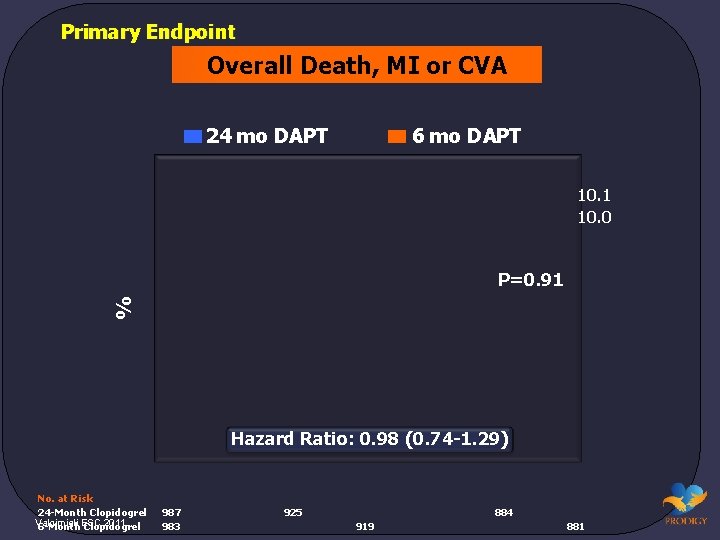
Primary Endpoint Overall Death, MI or CVA 24 mo DAPT 6 mo DAPT 10. 1 10. 0 % P=0. 91 Hazard Ratio: 0. 98 (0. 74 -1. 29) No. at Risk 24 -Month Clopidogrel Valgimigli 2011. 6 -Month. ESC Clopidogrel 987 983 925 884 919 881

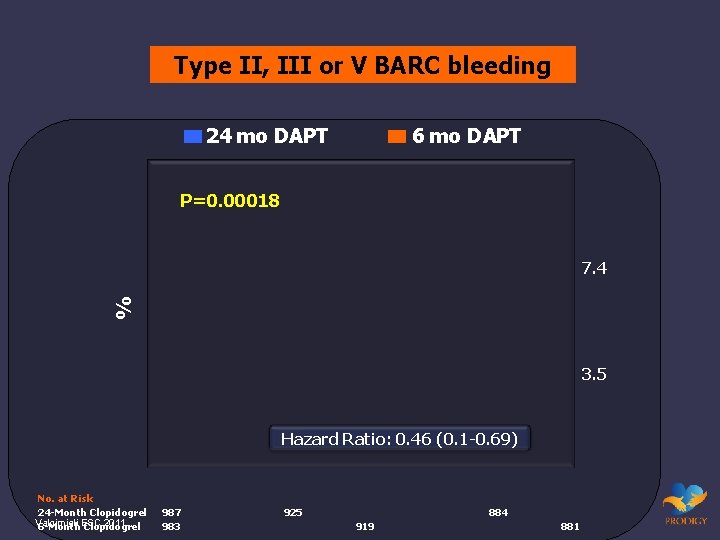
Type II, III or V BARC bleeding 24 mo DAPT 6 mo DAPT P=0. 00018 % 7. 4 3. 5 Hazard Ratio: 0. 46 (0. 1 -0. 69) No. at Risk 24 -Month Clopidogrel Valgimigli 2011. 6 -Month. ESC Clopidogrel 987 983 925 884 919 881

Bleeding endpoint in PRODIGY • Bleeding Academic Research Consortium* (BARC) definitions provide potential for uniform definition across trials and are hierarchical • Investigators changed bleeding endpoint from TIMI major to type II, IV BARC before final analysis • BARC type II includes any bleeding that triggers testing or treatment, even if no change in hemoglobin, blood transfusion or hemodynamic sequelae are present *Mehran et al. Circulation. 2011 Jun 14; 123(23): 2736 -47.

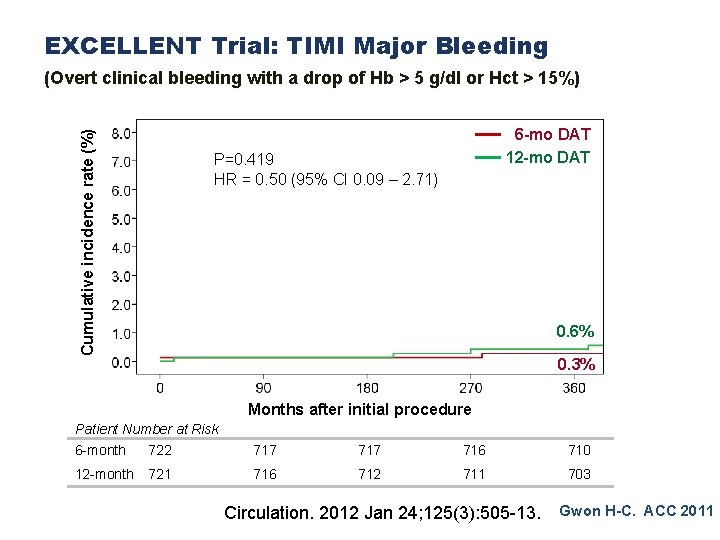
EXCELLENT Trial: TIMI Major Bleeding Cumulative incidence rate (%) (Overt clinical bleeding with a drop of Hb > 5 g/dl or Hct > 15%) 6 -mo DAT 12 -mo DAT P=0. 419 HR = 0. 50 (95% CI 0. 09 – 2. 71) 0. 6% 0. 3% Months after initial procedure Patient Number at Risk 6 -month 722 717 716 710 12 -month 721 716 712 711 703 Circulation. 2012 Jan 24; 125(3): 505 -13. Gwon H-C. ACC 2011

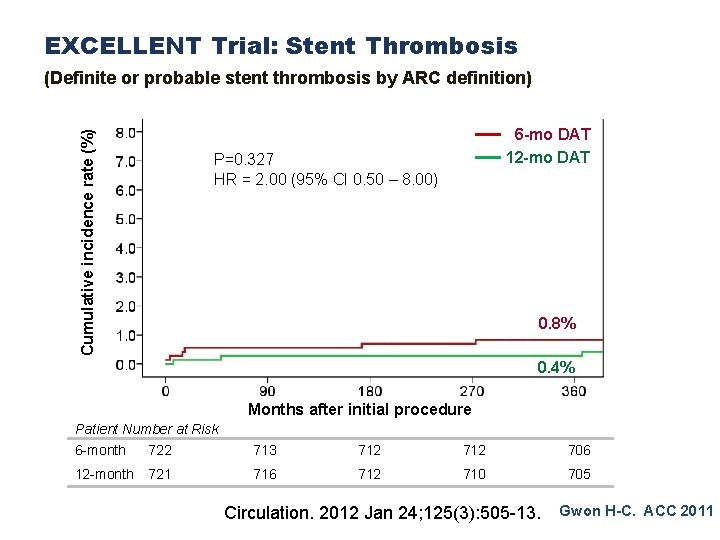
EXCELLENT Trial: Stent Thrombosis Cumulative incidence rate (%) (Definite or probable stent thrombosis by ARC definition) 6 -mo DAT 12 -mo DAT P=0. 327 HR = 2. 00 (95% CI 0. 50 – 8. 00) 0. 8% 0. 4% Months after initial procedure Patient Number at Risk 6 -month 722 713 712 706 12 -month 721 716 712 710 705 Circulation. 2012 Jan 24; 125(3): 505 -13. Gwon H-C. ACC 2011

Recent Studies of DAPT Duration in Context • Questions continue regarding benefit vs risk of longer thienopyridine therapy on MACCE • Recent study results have not been definitive • Not powered to determine differences in stent thrombosis • Variable treatment durations • Not blinded • Yet each of these studies highlights the remaining clinical question regarding DAPT: Is there a benefit (stent thrombosis or MACCE prevention) that outweighs the risk (bleeding) or cost

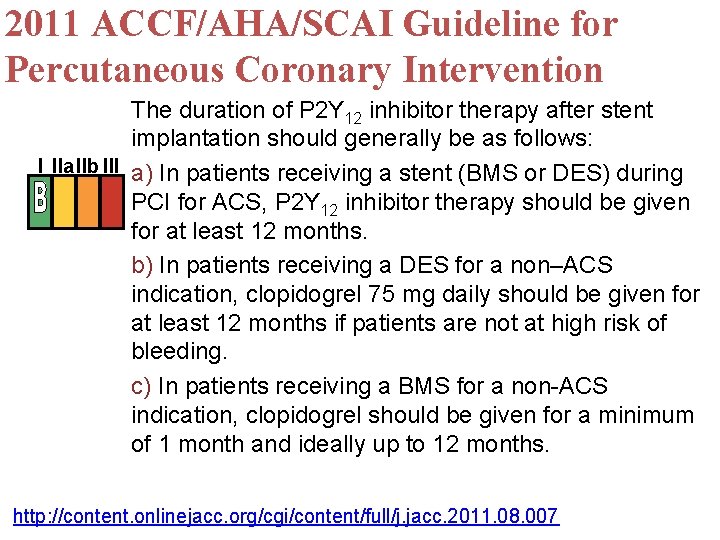
2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention The duration of P 2 Y 12 inhibitor therapy after stent implantation should generally be as follows: I IIa. IIb III a) In patients receiving a stent (BMS or DES) during PCI for ACS, P 2 Y 12 inhibitor therapy should be given for at least 12 months. b) In patients receiving a DES for a non–ACS indication, clopidogrel 75 mg daily should be given for at least 12 months if patients are not at high risk of bleeding. c) In patients receiving a BMS for a non-ACS indication, clopidogrel should be given for a minimum of 1 month and ideally up to 12 months. http: //content. onlinejacc. org/cgi/content/full/j. jacc. 2011. 08. 007

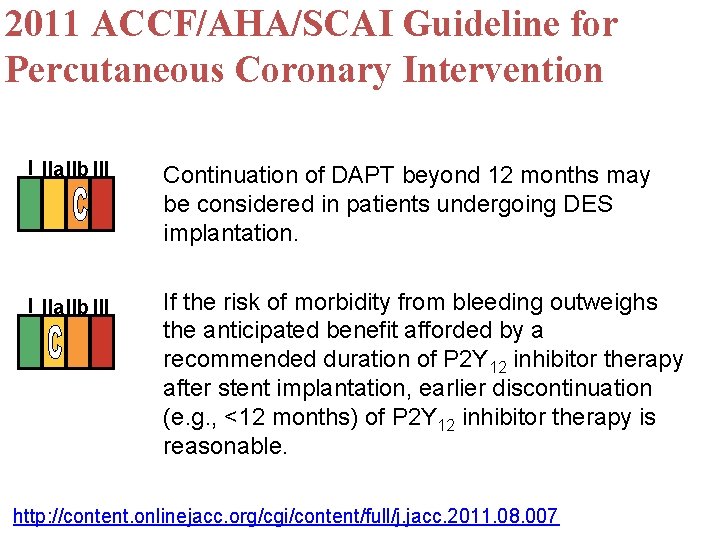
2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention I IIa. IIb III Continuation of DAPT beyond 12 months may be considered in patients undergoing DES implantation. I IIa. IIb III If the risk of morbidity from bleeding outweighs the anticipated benefit afforded by a recommended duration of P 2 Y 12 inhibitor therapy after stent implantation, earlier discontinuation (e. g. , <12 months) of P 2 Y 12 inhibitor therapy is reasonable. http: //content. onlinejacc. org/cgi/content/full/j. jacc. 2011. 08. 007

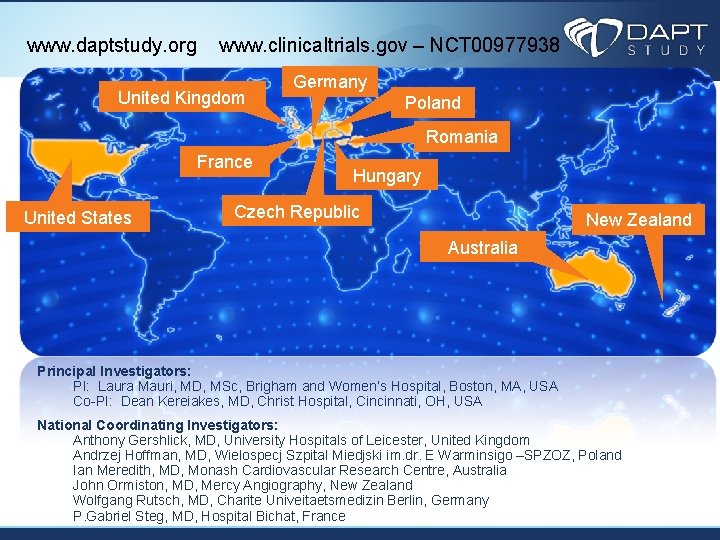
www. daptstudy. org www. clinicaltrials. gov – NCT 00977938 United Kingdom Germany Poland Romania France United States Hungary Czech Republic New Zealand Australia Principal Investigators: PI: Laura Mauri, MD, MSc, Brigham and Women’s Hospital, Boston, MA, USA Co-PI: Dean Kereiakes, MD, Christ Hospital, Cincinnati, OH, USA National Coordinating Investigators: Anthony Gershlick, MD, University Hospitals of Leicester, United Kingdom Andrzej Hoffman, MD, Wielospecj Szpital Miedjski im. dr. E Warminsigo –SPZOZ, Poland Ian Meredith, MD, Monash Cardiovascular Research Centre, Australia John Ormiston, MD, Mercy Angiography, New Zealand Wolfgang Rutsch, MD, Charite Univeitaetsmedizin Berlin, Germany P. Gabriel Steg, MD, Hospital Bichat, France

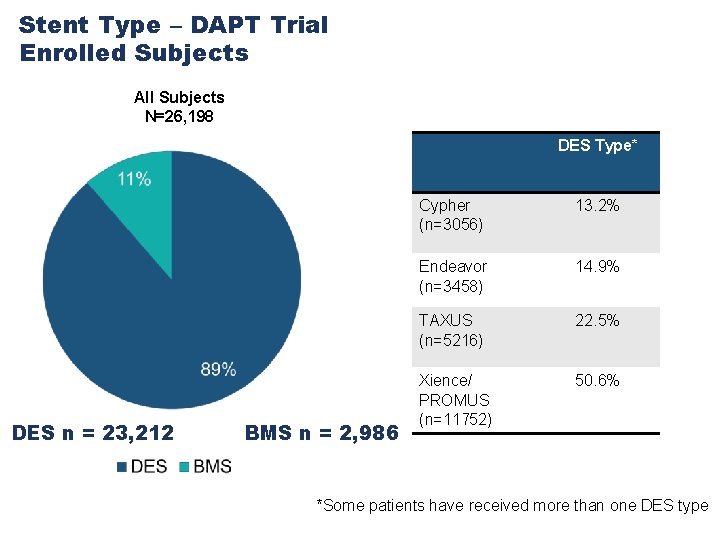
Total Subject Enrollment Don Cutlip, Harold Dauerman Daniel Simon, David Kandzari DES n = 23, 212 David Lee, Kirk Garratt James Hermiller, Mitch Krucoff Laura Mauri, Dean Kereiakes BMS n = 2, 986

Stent Type – DAPT Trial Enrolled Subjects All Subjects N=26, 198 DES Type* DES n = 23, 212 BMS n = 2, 986 Cypher (n=3056) 13. 2% Endeavor (n=3458) 14. 9% TAXUS (n=5216) 22. 5% Xience/ PROMUS (n=11752) 50. 6% *Some patients have received more than one DES type 23

Dual Antiplatelet Therapy After DES: When is it Safe to Stop? • Trials such as DAPT and ISAR SAFE are powered to answer this question • DAPT Study on track for results in 2014 • 26, 000+ enrolled • ~9000 subjects currently randomized • DSMB approval to continue • Until then, the current ACC AHA SCAI guideline of 12 m in most patients is reasonable