CRT 2012 DES InDepth NEW DES COMERS WITH

- Slides: 21

CRT 2012 DES In-Depth NEW DES COMERS WITH DUARABLE POLYMERS UPDATE Resolute DES is an investigational device, not FDA approved for use in the US Martin T Rothman VP Medical Affairs Coronary & Renal Denervation Medtronic Inc Professor of Interventional Cardiology Barts & The London NHS Trust Washington DC Feb 6, 2012

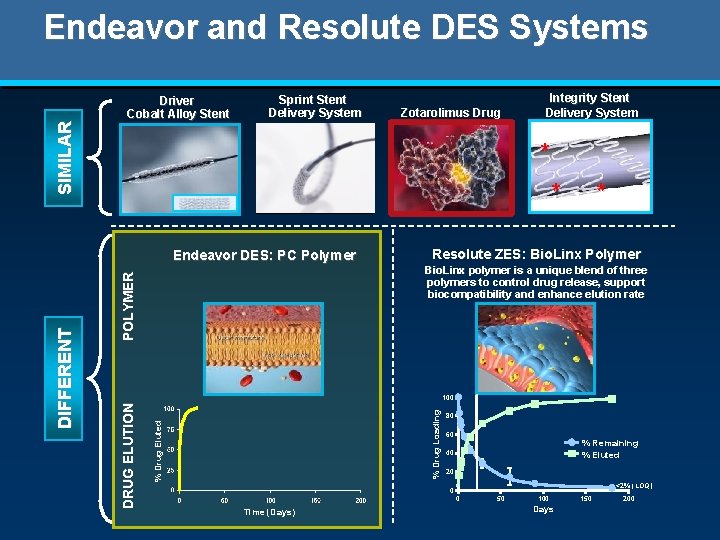
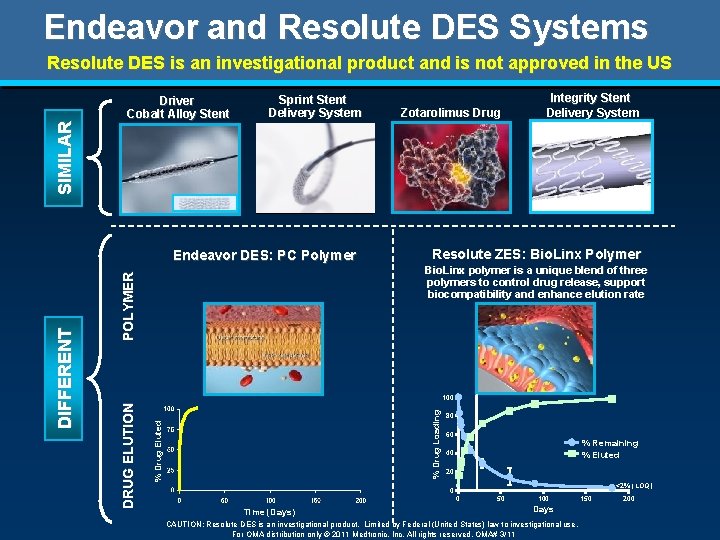
S I MI L A R Endeavor and Resolute DES Systems Driver Cobalt Alloy Stent Sprint Stent Delivery System * * Endeavor DES: PC Polymer * Resolute ZES: Bio. Linx Polymer P O L Y ME R Bio. Linx polymer is a unique blend of three polymers to control drug release, support biocompatibility and enhance elution rate % Drug Eluted % Drug Loading 100 DRUG E L UT I O N DI F F E RE NT Zotarolimus Drug Integrity Stent Delivery System 80 60 % Remaining % Eluted 40 20 <2% (LOQ) 0 0 Time (Days) 50 100 Days 150 200

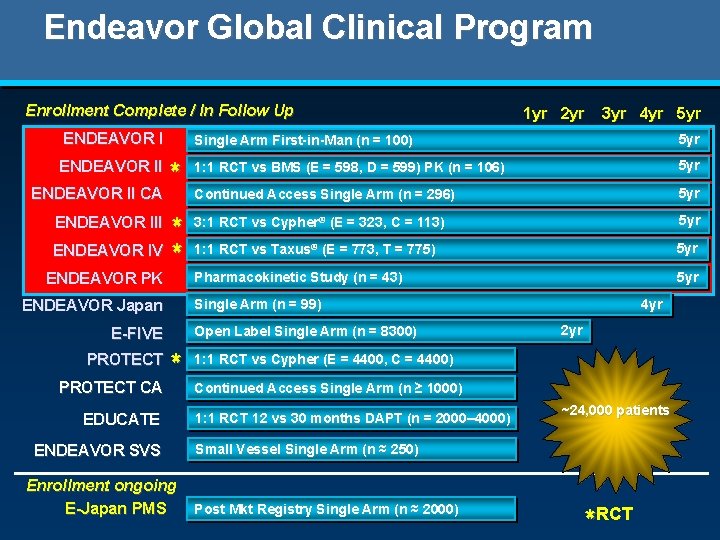
Endeavor Global Clinical Program Enrollment Complete / In Follow Up ENDEAVOR II CA * * ENDEAVOR IV * ENDEAVOR III ENDEAVOR PK ENDEAVOR Japan PROTECT CA 3 yr 4 yr 5 yr Single Arm First-in-Man (n = 100) 5 yr 1: 1 RCT vs BMS (E = 598, D = 599) PK (n = 106) 5 yr Continued Access Single Arm (n = 296) 5 yr 3: 1 RCT vs Cypher® (E = 323, C = 113) 5 yr 1: 1 RCT vs Taxus® (E = 773, T = 775) 5 yr Pharmacokinetic Study (n = 43) 5 yr Single Arm (n = 99) Open Label Single Arm (n = 8300) E-FIVE PROTECT 1 yr 2 yr * EDUCATE ENDEAVOR SVS Enrollment ongoing E-Japan PMS 4 yr 2 yr 1: 1 RCT vs Cypher (E = 4400, C = 4400) Continued Access Single Arm (n ≥ 1000) 1: 1 RCT 12 vs 30 months DAPT (n = 2000– 4000) ~24, 000 patients Small Vessel Single Arm (n ≈ 250) Post Mkt Registry Single Arm (n ≈ 2000) * RCT

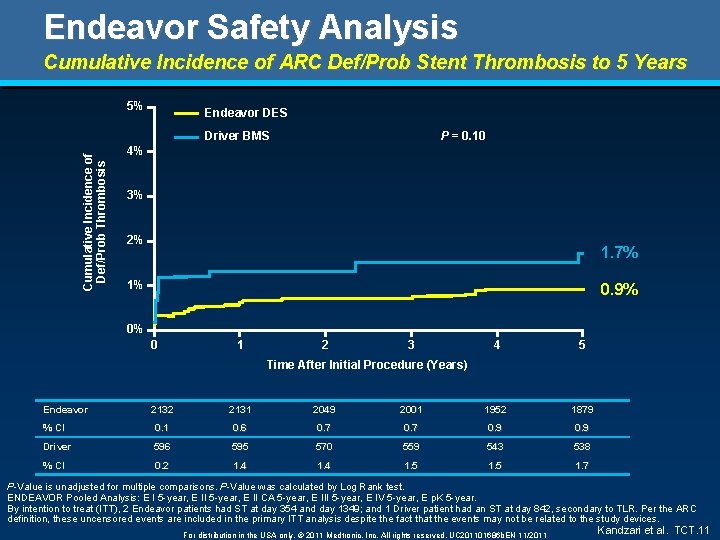
Endeavor Safety Analysis Cumulative Incidence of ARC Def/Prob Stent Thrombosis to 5 Years 5% Endeavor DES P = 0. 10 Cumulative Incidence of Def/Prob Thrombosis Driver BMS 4% 3% 2% 1. 7% 1% 0. 9% 0% 0 1 2 3 4 5 Time After Initial Procedure (Years) Endeavor 2132 2131 2049 2001 1952 1879 % CI 0. 1 0. 6 0. 7 0. 9 Driver 596 595 570 559 543 538 % CI 0. 2 1. 4 1. 5 1. 7 P-Value is unadjusted for multiple comparisons. P-Value was calculated by Log Rank test. ENDEAVOR Pooled Analysis: E I 5 -year, E II CA 5 -year, E III 5 -year, E IV 5 -year, E p. K 5 -year. By intention to treat (ITT), 2 Endeavor patients had ST at day 354 and day 1349; and 1 Driver patient had an ST at day 842, secondary to TLR. Per the ARC definition, these uncensored events are included in the primary ITT analysis despite the fact that the events may not be related to the study devices. For distribution in the USA only. © 2011 Medtronic, Inc. All rights reserved. UC 201101686 b. EN 11/2011 Kandzari et al. TCT. 11

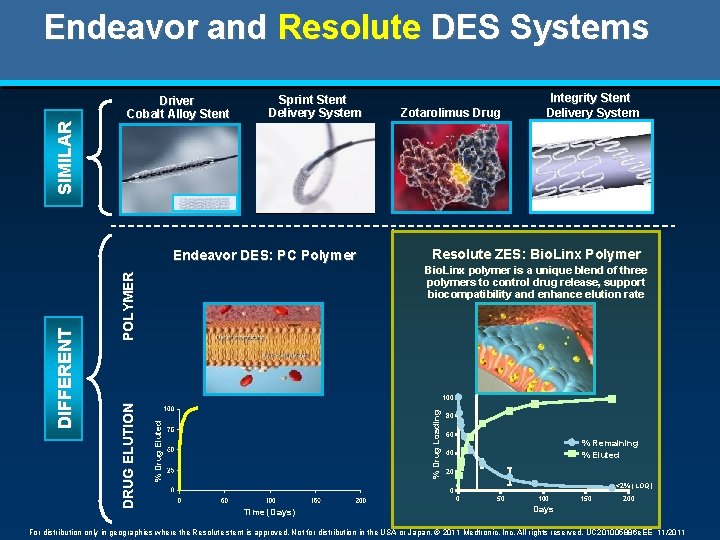
S I MI L A R Endeavor and Resolute DES Systems Driver Cobalt Alloy Stent Sprint Stent Delivery System Resolute ZES: Bio. Linx Polymer P O L Y ME R Bio. Linx polymer is a unique blend of three polymers to control drug release, support biocompatibility and enhance elution rate % Drug Eluted % Drug Loading 100 DRUG E L UT I O N DI F F E RE NT Endeavor DES: PC Polymer Zotarolimus Drug Integrity Stent Delivery System 80 60 % Remaining % Eluted 40 20 <2% (LOQ) 0 0 Time (Days) 50 100 150 200 Days For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

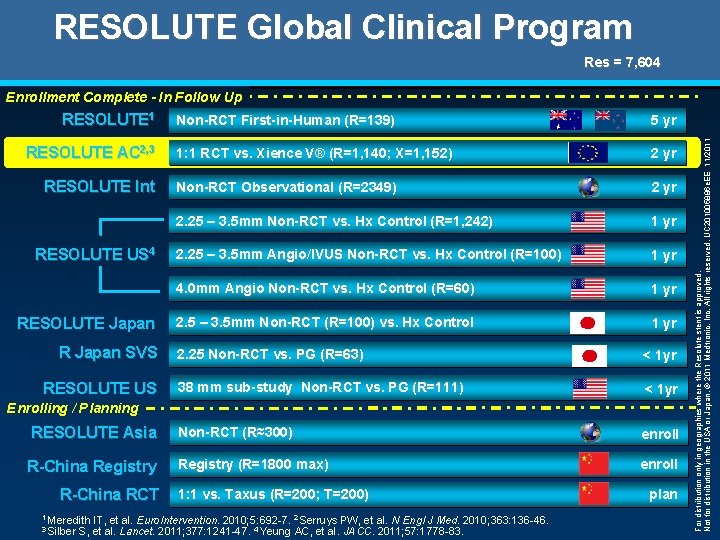
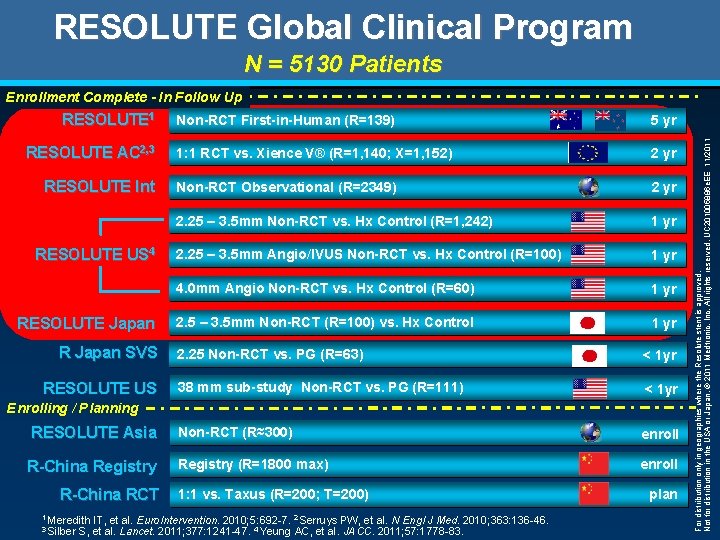
RESOLUTE Global Clinical Program Res = 7, 604 RESOLUTE 1 RESOLUTE AC 2, 3 RESOLUTE Int RESOLUTE US 4 RESOLUTE Japan R Japan SVS RESOLUTE US Non-RCT First-in-Human (R=139) 5 yr 1: 1 RCT vs. Xience V® (R=1, 140; X=1, 152) 2 yr Non-RCT Observational (R=2349) 2 yr 2. 25 – 3. 5 mm Non-RCT vs. Hx Control (R=1, 242) 1 yr 2. 25 – 3. 5 mm Angio/IVUS Non-RCT vs. Hx Control (R=100) 1 yr 4. 0 mm Angio Non-RCT vs. Hx Control (R=60) 1 yr 2. 5 – 3. 5 mm Non-RCT (R=100) vs. Hx Control 1 yr 2. 25 Non-RCT vs. PG (R=63) < 1 yr 38 mm sub-study Non-RCT vs. PG (R=111) < 1 yr Non-RCT (R≈300) enroll Registry (R=1800 max) enroll Enrolling / Planning RESOLUTE Asia R-China Registry R-China RCT 1 Meredith 3 Silber 1: 1 vs. Taxus (R=200; T=200) IT, et al. Euro. Intervention. 2010; 5: 692 -7. 2 Serruys PW, et al. N Engl J Med. 2010; 363: 136 -46. S, et al. Lancet. 2011; 377: 1241 -47. 4 Yeung AC, et al. JACC. 2011; 57: 1778 -83. plan For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011 Enrollment Complete - In Follow Up

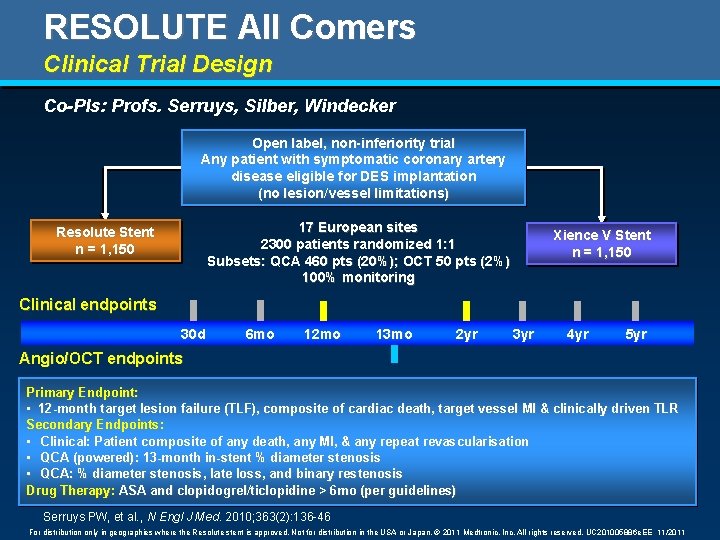
RESOLUTE All Comers Clinical Trial Design Co-PIs: Profs. Serruys, Silber, Windecker Open label, non-inferiority trial Any patient with symptomatic coronary artery disease eligible for DES implantation (no lesion/vessel limitations) 17 European sites 2300 patients randomized 1: 1 Subsets: QCA 460 pts (20%); OCT 50 pts (2%) 100% monitoring Resolute Stent n = 1, 150 Xience V Stent n = 1, 150 Clinical endpoints 30 d 6 mo 12 mo 13 mo 2 yr 3 yr 4 yr 5 yr Angio/OCT endpoints Primary Endpoint: • 12 -month target lesion failure (TLF), composite of cardiac death, target vessel MI & clinically driven TLR Secondary Endpoints: • Clinical: Patient composite of any death, any MI, & any repeat revascularisation • QCA (powered): 13 -month in-stent % diameter stenosis • QCA: % diameter stenosis, late loss, and binary restenosis Drug Therapy: ASA and clopidogrel/ticlopidine > 6 mo (per guidelines) Serruys PW, et al. , N Engl J Med. 2010; 363(2): 136 -46 For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

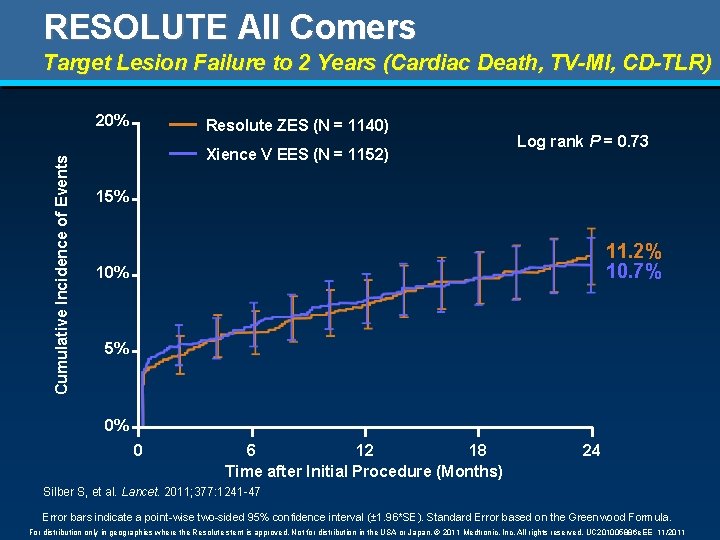
RESOLUTE All Comers Target Lesion Failure to 2 Years (Cardiac Death, TV-MI, CD-TLR) Cumulative Incidence of Events 20% Resolute ZES (N = 1140) Xience V EES (N = 1152) Log rank P = 0. 73 15% 11. 2% 10. 7% 10% 5% 0% 0 6 12 18 Time after Initial Procedure (Months) 24 Silber S, et al. Lancet. 2011; 377: 1241 -47 Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula. For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

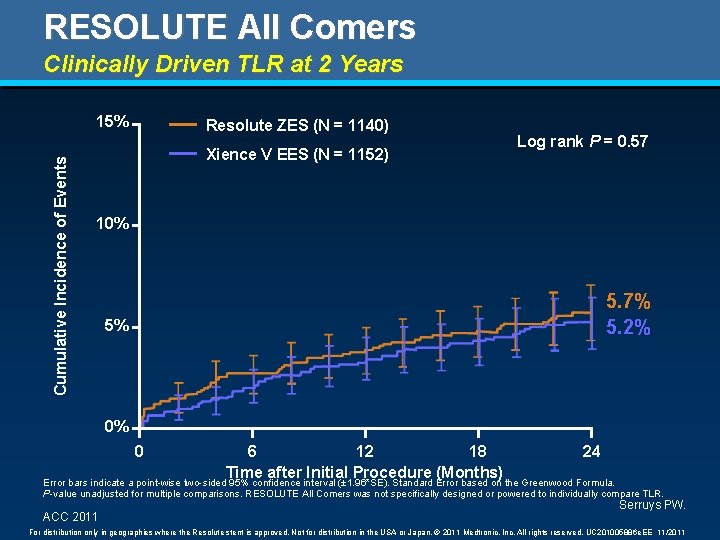
RESOLUTE All Comers Clinically Driven TLR at 2 Years Cumulative Incidence of Events 15% Resolute ZES (N = 1140) Xience V EES (N = 1152) Log rank P = 0. 57 10% 5. 7% 5. 2% 5% 0% 0 6 12 18 24 Time after Initial Procedure (Months) Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula. P-value unadjusted for multiple comparisons. RESOLUTE All Comers was not specifically designed or powered to individually compare TLR. ACC 2011 Serruys PW. For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

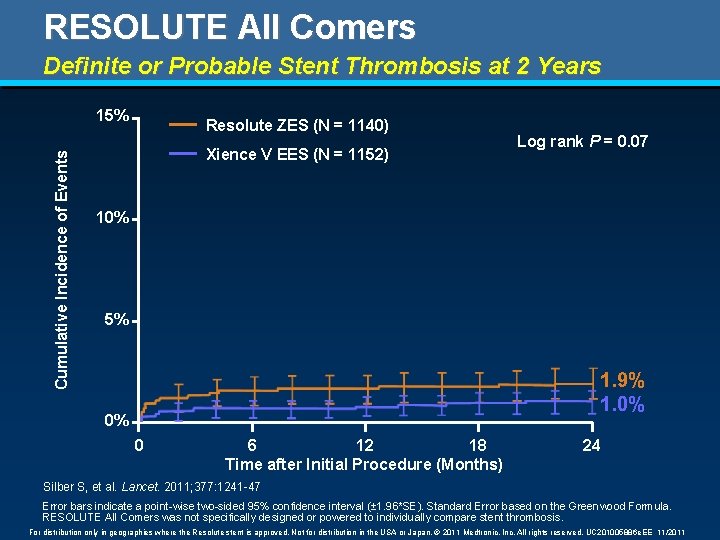
RESOLUTE All Comers Definite or Probable Stent Thrombosis at 2 Years Cumulative Incidence of Events 15% Resolute ZES (N = 1140) Xience V EES (N = 1152) Log rank P = 0. 07 10% 5% 1. 9% 1. 0% 0% 0 6 12 18 Time after Initial Procedure (Months) 24 Silber S, et al. Lancet. 2011; 377: 1241 -47 Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula. RESOLUTE All Comers was not specifically designed or powered to individually compare stent thrombosis. For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

RESOLUTE Global Clinical Program N = 5130 Patients RESOLUTE 1 RESOLUTE AC 2, 3 RESOLUTE Int RESOLUTE US 4 RESOLUTE Japan R Japan SVS RESOLUTE US Non-RCT First-in-Human (R=139) 5 yr 1: 1 RCT vs. Xience V® (R=1, 140; X=1, 152) 2 yr Non-RCT Observational (R=2349) 2 yr 2. 25 – 3. 5 mm Non-RCT vs. Hx Control (R=1, 242) 1 yr 2. 25 – 3. 5 mm Angio/IVUS Non-RCT vs. Hx Control (R=100) 1 yr 4. 0 mm Angio Non-RCT vs. Hx Control (R=60) 1 yr 2. 5 – 3. 5 mm Non-RCT (R=100) vs. Hx Control 1 yr 2. 25 Non-RCT vs. PG (R=63) < 1 yr 38 mm sub-study Non-RCT vs. PG (R=111) < 1 yr Non-RCT (R≈300) enroll Registry (R=1800 max) enroll Enrolling / Planning RESOLUTE Asia R-China Registry R-China RCT 1 Meredith 3 Silber 1: 1 vs. Taxus (R=200; T=200) IT, et al. Euro. Intervention. 2010; 5: 692 -7. 2 Serruys PW, et al. N Engl J Med. 2010; 363: 136 -46. S, et al. Lancet. 2011; 377: 1241 -47. 4 Yeung AC, et al. JACC. 2011; 57: 1778 -83. plan For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011 Enrollment Complete - In Follow Up

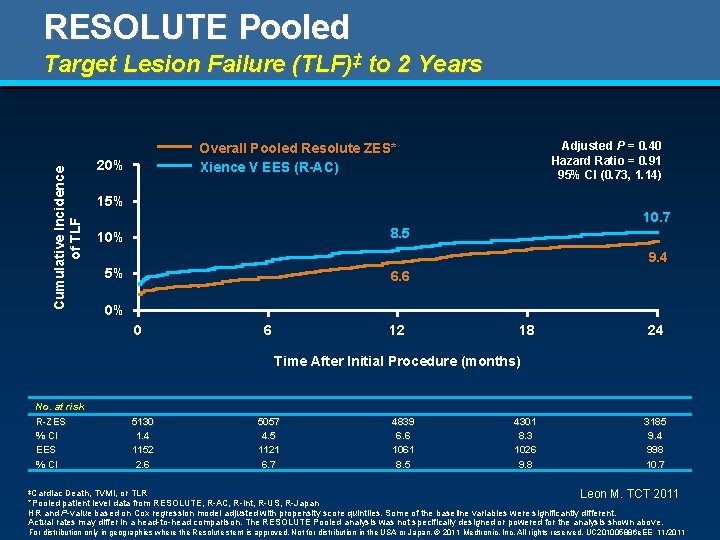
RESOLUTE Pooled Cumulative Incidence of TLF Target Lesion Failure (TLF)‡ to 2 Years Adjusted P = 0. 40 Hazard Ratio = 0. 91 95% CI (0. 73, 1. 14) Overall Pooled Resolute ZES* Xience V EES (R-AC) 20% 15% 10. 7 8. 5 10% 9. 4 5% 6. 6 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI EES % CI 5130 1. 4 1152 2. 6 5057 4. 5 1121 6. 7 4839 6. 6 1061 8. 5 4301 8. 3 1026 9. 8 3185 9. 4 998 10. 7 Death, TVMI, or TLR Leon M. TCT 2011 *Pooled patient level data from RESOLUTE, R-AC, R-Int, R-US, R-Japan HR and P-value based on Cox regression model adjusted with propensity score quintiles. Some of the baseline variables were significantly different. Actual rates may differ in a head-to-head comparison. The RESOLUTE Pooled analysis was not specifically designed or powered for the analysis shown above. ‡Cardiac For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

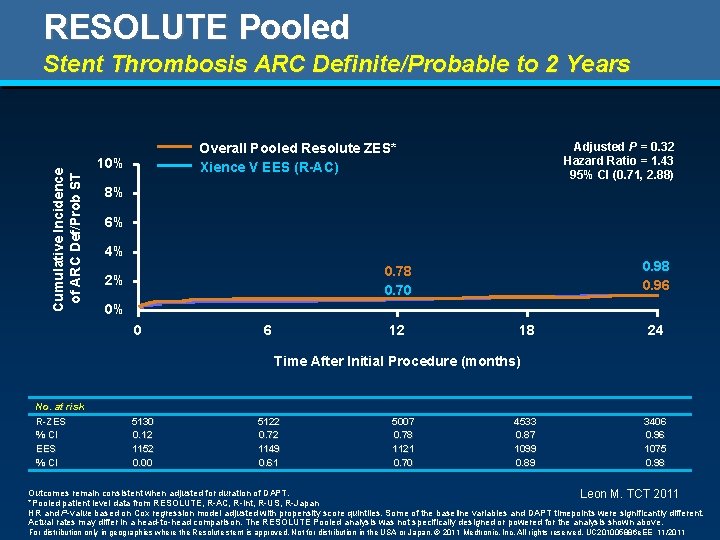
RESOLUTE Pooled Cumulative Incidence of ARC Def/Prob ST Stent Thrombosis ARC Definite/Probable to 2 Years Adjusted P = 0. 32 Hazard Ratio = 1. 43 95% CI (0. 71, 2. 88) Overall Pooled Resolute ZES* Xience V EES (R-AC) 10% 8% 6% 4% 0. 98 0. 96 0. 78 0. 70 2% 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI EES % CI 5130 0. 12 1152 0. 00 5122 0. 72 1149 0. 61 5007 0. 78 1121 0. 70 4533 0. 87 1099 0. 89 3406 0. 96 1075 0. 98 Outcomes remain consistent when adjusted for duration of DAPT. Leon M. TCT 2011 *Pooled patient level data from RESOLUTE, R-AC, R-Int, R-US, R-Japan HR and P-value based on Cox regression model adjusted with propensity score quintiles. Some of the baseline variables and DAPT timepoints were significantly different. Actual rates may differ in a head-to-head comparison. The RESOLUTE Pooled analysis was not specifically designed or powered for the analysis shown above. For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

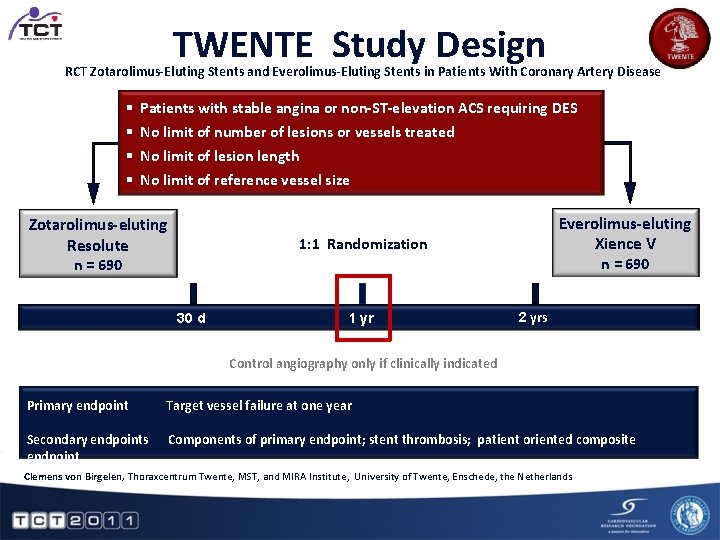
TWENTE Study Design RCT Zotarolimus-Eluting Stents and Everolimus-Eluting Stents in Patients With Coronary Artery Disease § § Patients with stable angina or non-ST-elevation ACS requiring DES No limit of number of lesions or vessels treated No limit of lesion length No limit of reference vessel size Zotarolimus-eluting Resolute Everolimus-eluting Xience V 1: 1 Randomization n = 690 30 d 1 yr 2 yrs Clinical follow-up Control angiography only if clinically indicated Primary endpoint Target vessel failure at one year Secondary endpoints endpoint Components of primary endpoint; stent thrombosis; patient oriented composite Clemens von Birgelen, Thoraxcentrum Twente, MST, and MIRA Institute, University of Twente, Enschede, the Netherlands

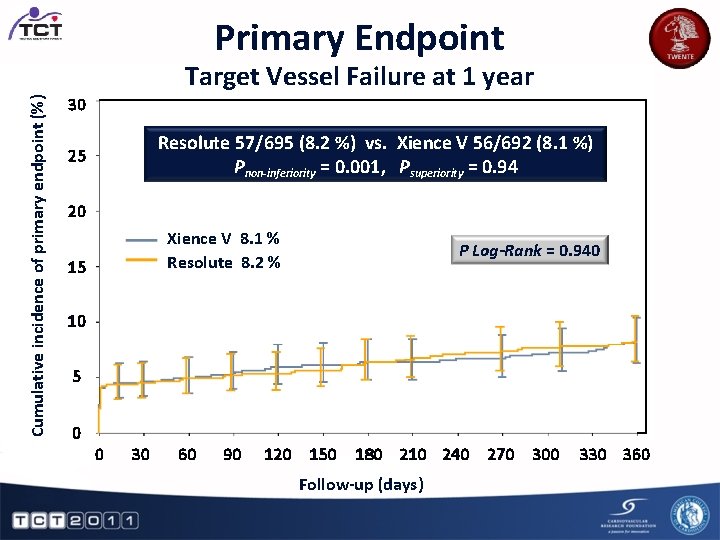
Primary Endpoint Cumulative incidence of primary endpoint (%) Target Vessel Failure at 1 year 30 Resolute 57/695 (8. 2 %) vs. Xience V 56/692 (8. 1 %) Pnon-inferiority = 0. 001, Psuperiority = 0. 94 25 20 Xience V 8. 1 % Resolute 8. 2 % 15 P Log-Rank = 0. 940 10 5 0 0 30 60 90 120 150 180 210 240 270 300 Follow-up (days) 330 360

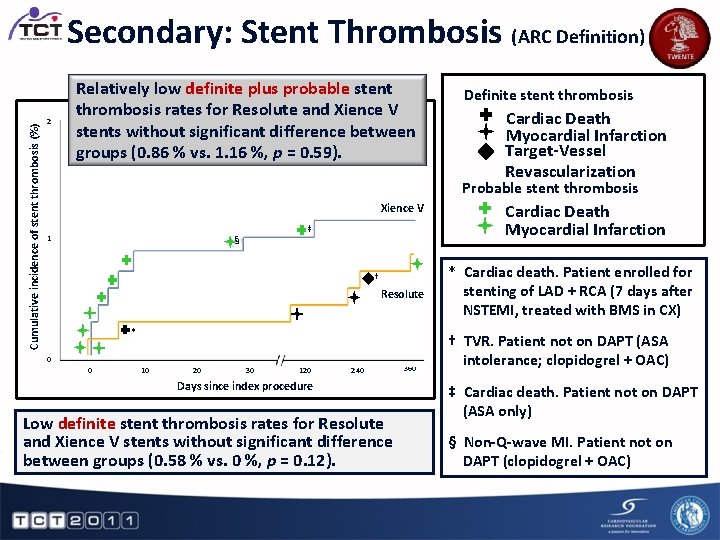
Cumulative incidence of stent thrombosis (%) Secondary: Stent Thrombosis (ARC Definition) 2 Relatively low definite plus probable stent thrombosis rates for Resolute and Xience V stents without significant difference between Definite Stent Thrombosis Probable Stent Thrombosis groups (0. 86 % vs. 1. 16 %, p Cardiac = 0. 59). Cardiac Death Target-Vessel Revascularization 1 Xience V ‡ § † Resolute * 0 0 10 20 30 120 Cardiac Death Myocardial Infarction Target-Vessel Revascularization Probable stent thrombosis Myocardial Infarction Definite stent thrombosis 240 Days since index procedure Low definite stent thrombosis rates for Resolute and Xience V stents without significant difference between groups (0. 58 % vs. 0 %, p = 0. 12). 360 Cardiac Death Myocardial Infarction * Cardiac death. Patient enrolled for stenting of LAD + RCA (7 days after NSTEMI, treated with BMS in CX) † TVR. Patient not on DAPT (ASA intolerance; clopidogrel + OAC) ‡ Cardiac death. Patient not on DAPT (ASA only) § Non-Q-wave MI. Patient not on DAPT (clopidogrel + OAC)

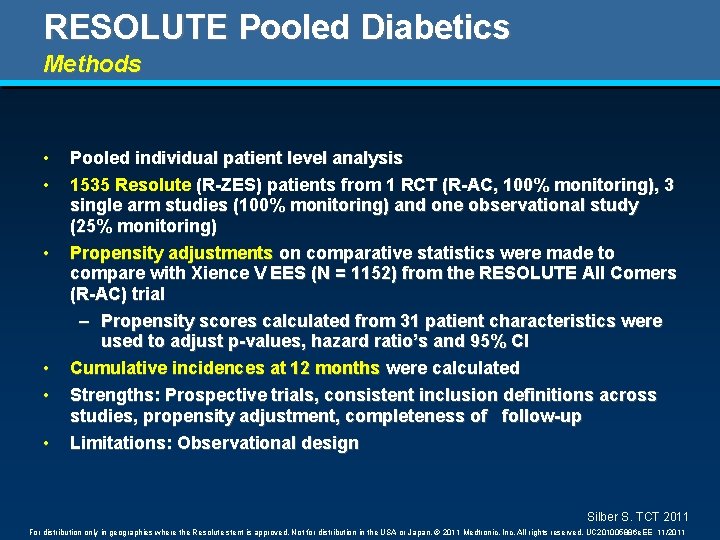
RESOLUTE Pooled Diabetics Methods • • • Pooled individual patient level analysis 1535 Resolute (R-ZES) patients from 1 RCT (R-AC, 100% monitoring), 3 single arm studies (100% monitoring) and one observational study (25% monitoring) Propensity adjustments on comparative statistics were made to compare with Xience V EES (N = 1152) from the RESOLUTE All Comers (R-AC) trial – Propensity scores calculated from 31 patient characteristics were used to adjust p-values, hazard ratio’s and 95% CI Cumulative incidences at 12 months were calculated Strengths: Prospective trials, consistent inclusion definitions across studies, propensity adjustment, completeness of follow-up Limitations: Observational design Silber S. TCT 2011 For distribution only in geographies where the Resolute stent is approved. Not for distribution in the USA or Japan. © 2011 Medtronic, Inc. All rights reserved. UC 201005886 e. EE 11/2011

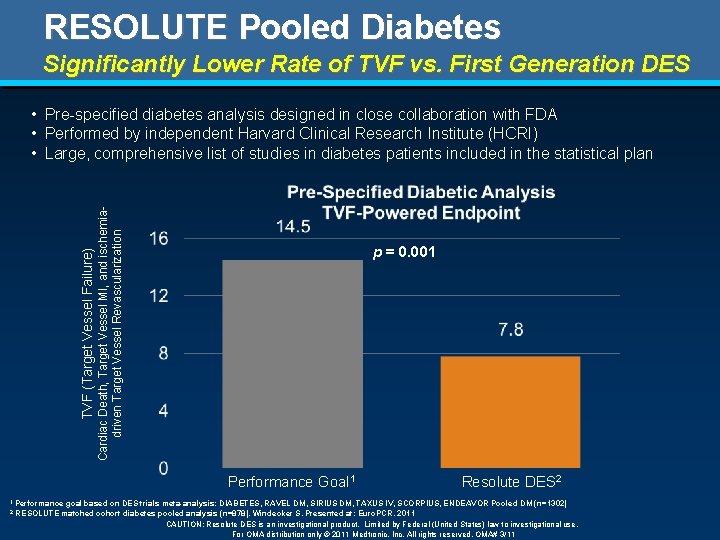
RESOLUTE Pooled Diabetes Significantly Lower Rate of TVF vs. First Generation DES TVF (Target Vessel Failure) Cardiac Death, Target Vessel MI, and ischemiadriven Target Vessel Revascularization • Pre-specified diabetes analysis designed in close collaboration with FDA • Performed by independent Harvard Clinical Research Institute (HCRI) • Large, comprehensive list of studies in diabetes patients included in the statistical plan p = 0. 001 Performance Goal 1 1 Performance 2 Resolute DES 2 goal based on DES trials meta-analysis: DIABETES, RAVEL DM, SIRIUS DM, TAXUS IV, SCORPIUS, ENDEAVOR Pooled DM (n=1302) RESOLUTE matched cohort diabetes pooled analysis (n=878). Windecker S. Presented at: Euro. PCR. 2011 CAUTION: Resolute DES is an investigational product. Limited by Federal (United States) law to investigational use. For OMA distribution only © 2011 Medtronic, Inc. All rights reserved. OMA# 3/11

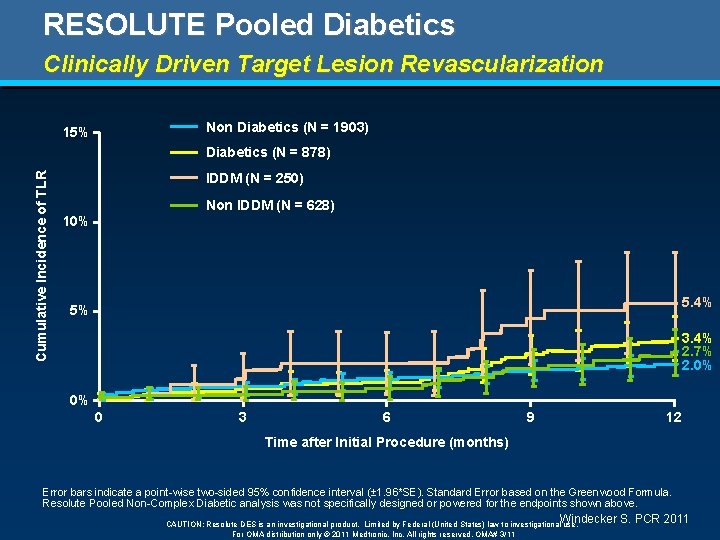
RESOLUTE Pooled Diabetics Clinically Driven Target Lesion Revascularization Non Diabetics (N = 1903) 15% Cumulative Incidence of TLR Diabetics (N = 878) IDDM (N = 250) Non IDDM (N = 628) 10% 5. 4% 5% 3. 4% 2. 7% 2. 0% 0% 0 3 6 9 12 Time after Initial Procedure (months) Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula. Resolute Pooled Non-Complex Diabetic analysis was not specifically designed or powered for the endpoints shown above. Windecker S. PCR 2011 CAUTION: Resolute DES is an investigational product. Limited by Federal (United States) law to investigational use. For OMA distribution only © 2011 Medtronic, Inc. All rights reserved. OMA# 3/11

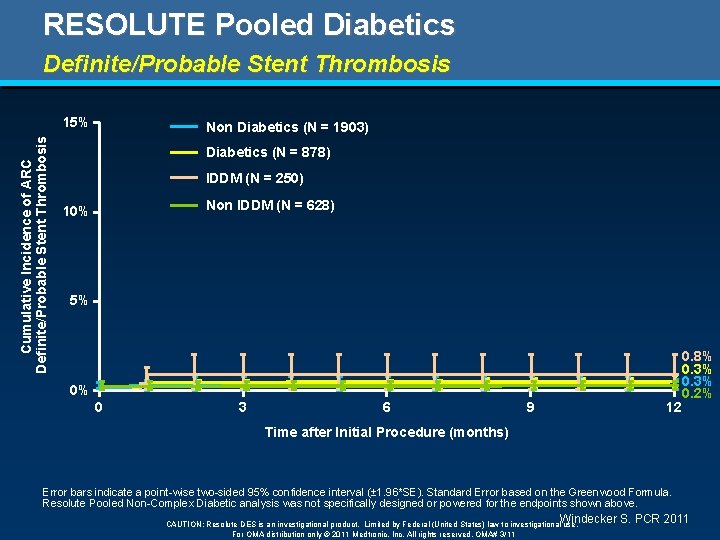
RESOLUTE Pooled Diabetics Definite/Probable Stent Thrombosis Cumulative Incidence of ARC Definite/Probable Stent Thrombosis 15% Non Diabetics (N = 1903) Diabetics (N = 878) IDDM (N = 250) Non IDDM (N = 628) 10% 5% 0% 0 3 6 9 12 0. 8% 0. 3% 0. 2% Time after Initial Procedure (months) Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula. Resolute Pooled Non-Complex Diabetic analysis was not specifically designed or powered for the endpoints shown above. Windecker S. PCR 2011 CAUTION: Resolute DES is an investigational product. Limited by Federal (United States) law to investigational use. For OMA distribution only © 2011 Medtronic, Inc. All rights reserved. OMA# 3/11

Endeavor and Resolute DES Systems S I MI L A R Resolute DES is an investigational product and is not approved in the US Driver Cobalt Alloy Stent Sprint Stent Delivery System Resolute ZES: Bio. Linx Polymer P O L Y ME R Bio. Linx polymer is a unique blend of three polymers to control drug release, support biocompatibility and enhance elution rate % Drug Eluted % Drug Loading 100 DRUG E L UT I O N DI F F E RE NT Endeavor DES: PC Polymer Zotarolimus Drug Integrity Stent Delivery System 80 60 % Remaining % Eluted 40 20 <2% (LOQ) 0 0 Time (Days) 50 100 Days CAUTION: Resolute DES is an investigational product. Limited by Federal (United States) law to investigational use. For OMA distribution only © 2011 Medtronic, Inc. All rights reserved. OMA# 3/11 150 200