Core Topic 8 Storage and handling of vaccine















- Slides: 23

Core Topic 8 Storage and handling of vaccine

Learning Outcome To follow correct procedures for storage and handling of vaccines Immunisation Department, Centre for Infections

Learning Objectives • Describe the cold chain and the importance of its maintenance • Specify minimum/maximum temperatures for vaccine storage • Describe the effects of temperature on potency and efficacy of vaccine • Describe the requirements for the correct ordering, delivery and storage of vaccines in the workplace • Identify vaccines sensitive to light heat and freezing • Know how to manage breakdowns in the cold chain, where to dispose of damaged vaccine, who to inform and what action to take • Explain how to audit current management of cold chain within their practice area. Immunisation Department, Centre for Infections

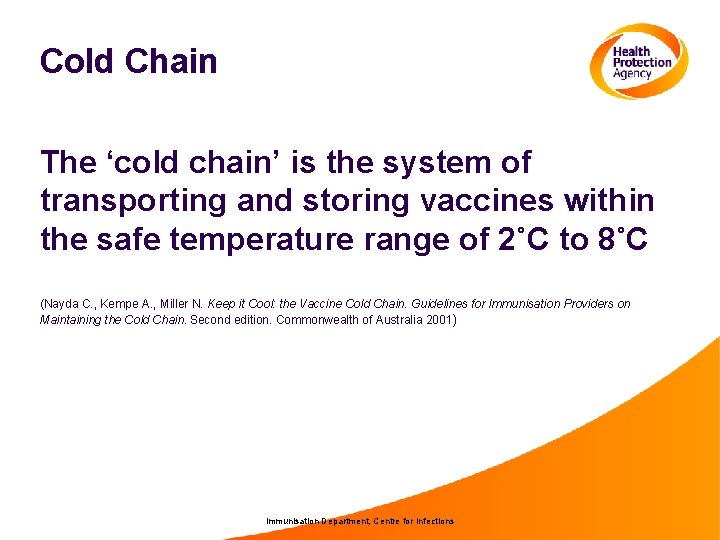
Cold Chain The ‘cold chain’ is the system of transporting and storing vaccines within the safe temperature range of 2˚C to 8˚C (Nayda C. , Kempe A. , Miller N. Keep it Cool: the Vaccine Cold Chain. Guidelines for Immunisation Providers on Maintaining the Cold Chain. Second edition. Commonwealth of Australia 2001) Immunisation Department, Centre for Infections

Why is the cold chain important? 1. Vaccines are: Ø Biological products Ø lose potency with time Ø Process irreversible and accelerated if proper storage conditions are not adhered to. Immunisation Department, Centre for Infections

Why is the cold chain important? 2. Assurance/confidence in potent product and vaccine programmes Ø Professional responsibility ü Confident the vaccines you give will be effective Ø Public Health responsibility ü Public confidence in immunisation programmes Immunisation Department, Centre for Infections

Why is the cold chain important? 3. Ensuring maximum benefit from immunisations ØResponsibility not to waste scarce NHS resources ØReduce wastage from errors Immunisation Department, Centre for Infections

Vaccine costs and wastage The Department of Health spends over £ 150 million a year on vaccines for the routine childhood programme, all of which are provided free of charge to GP surgeries and PCTs The cost to provide vaccines for the completion of a routine childhood immunisation schedule to an individual child up to the age of 16 is over £ 200 (Vaccine Update Jan 2008) This dose not include a 3 dose course of HPV (Human Papillomavirus) vaccine! Immunisation Department, Centre for Infections

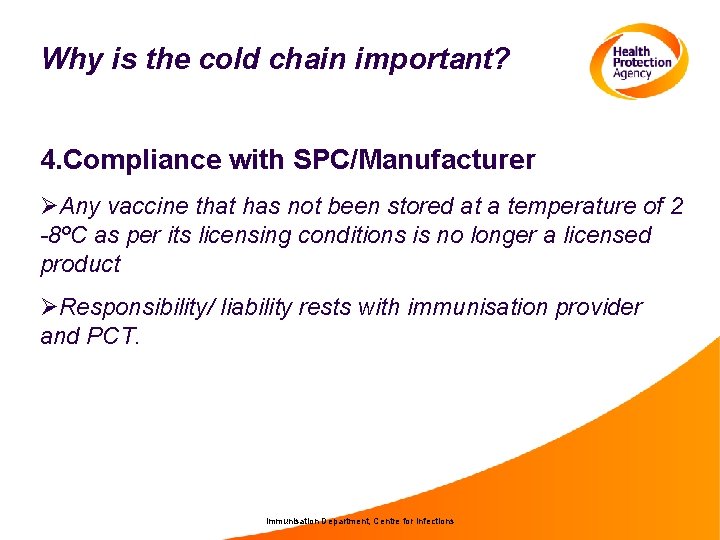
Why is the cold chain important? 4. Compliance with SPC/Manufacturer ØAny vaccine that has not been stored at a temperature of 2 -8ºC as per its licensing conditions is no longer a licensed product ØResponsibility/ liability rests with immunisation provider and PCT. Immunisation Department, Centre for Infections

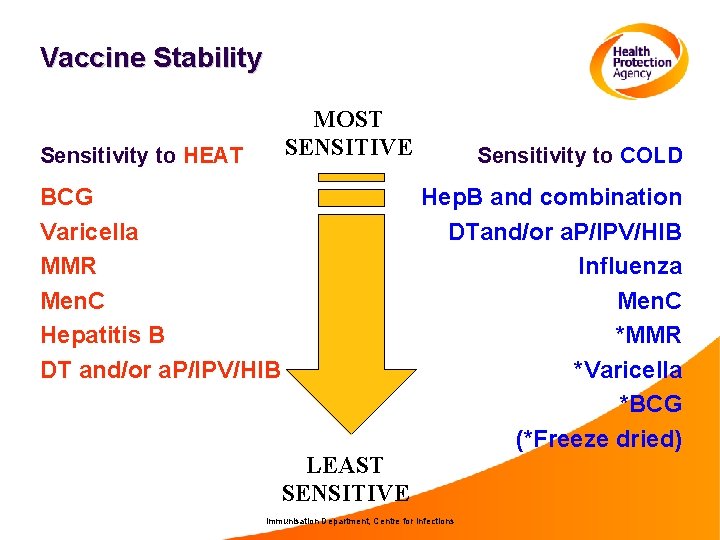
Vaccine Stability MOST SENSITIVE Sensitivity to HEAT BCG Varicella MMR Men. C Hepatitis B DT and/or a. P/IPV/HIB LEAST SENSITIVE Sensitivity to COLD Hep. B and combination DTand/or a. P/IPV/HIB Influenza Men. C *MMR *Varicella *BCG (*Freeze dried) Immunisation Department, Centre for Infections

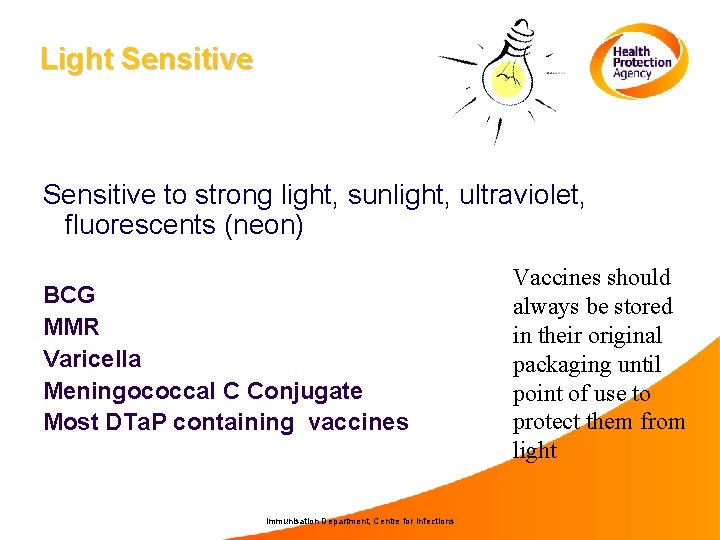
Light Sensitive to strong light, sunlight, ultraviolet, fluorescents (neon) BCG MMR Varicella Meningococcal C Conjugate Most DTa. P containing vaccines Immunisation Department, Centre for Infections Vaccines should always be stored in their original packaging until point of use to protect them from light

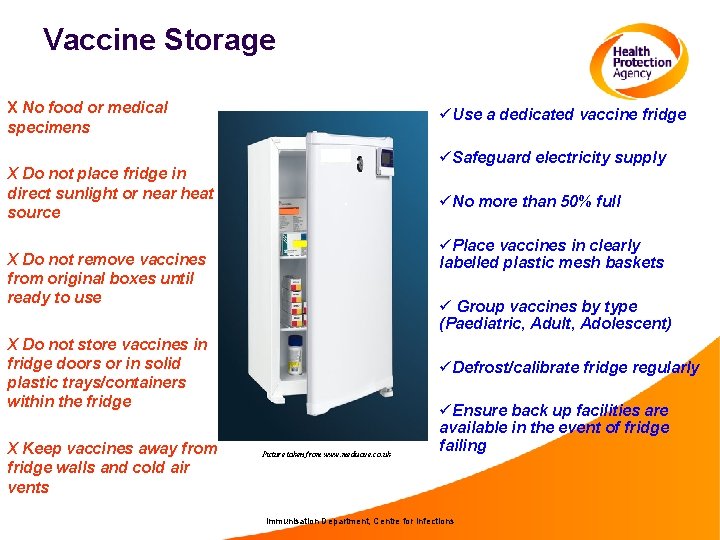
Vaccine Storage X No food or medical specimens üUse a dedicated vaccine fridge üSafeguard electricity supply X Do not place fridge in direct sunlight or near heat source üNo more than 50% full üPlace vaccines in clearly labelled plastic mesh baskets X Do not remove vaccines from original boxes until ready to use ü Group vaccines by type (Paediatric, Adult, Adolescent) X Do not store vaccines in fridge doors or in solid plastic trays/containers within the fridge X Keep vaccines away from fridge walls and cold air vents üDefrost/calibrate fridge regularly Picture taken from www. medisave. co. uk üEnsure back up facilities are available in the event of fridge failing Immunisation Department, Centre for Infections

Temperature Monitoring ü Use max/min thermometer üProbe should be placed in the centre of fridge üTemperature should be recorded at least once a day üReset daily üCalibrate as recommended üTake immediate action if temperature is outside recommended range Immunisation Department, Centre for Infections

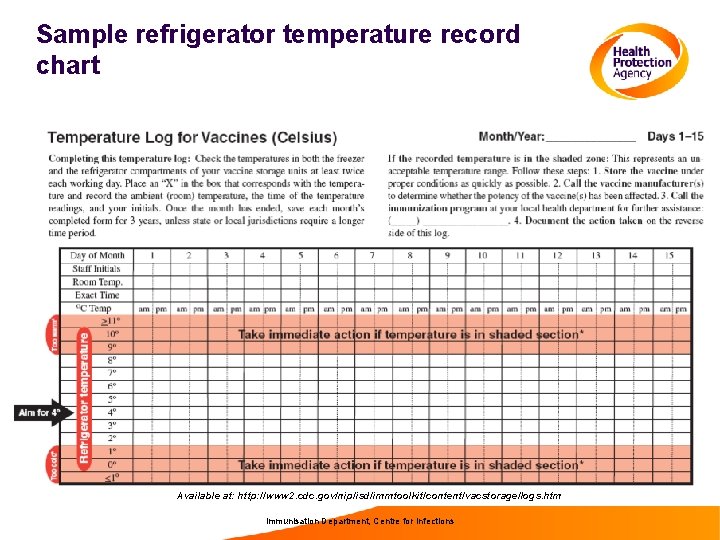
Sample refrigerator temperature record chart Available at: http: //www 2. cdc. gov/nip/isd/immtoolkit/content/vacstorage/logs. htm Immunisation Department, Centre for Infections

Storage temperature Never exceed 8ºC or fall below 2ºC Aim for 5ºC Aim to maintain vaccine fridge as close as possible to 5˚C as this gives a safety margin of + or – 3˚c Immunisation Department, Centre for Infections

Ordering and Delivery Named trained designated person and deputy who have overall responsibility for ordering, receipt and care of vaccines. Responsibilities include: ØEnsuring cold chain has been maintained during transport and managing receipt of vaccines directly into refrigeration ØChecking delivery for leakage, damage and discrepancies Ø Rotation of stock ØMaintaining stock information system to keep track of orders, expiry dates and running total of vaccines ØEnsuring adequate supply/ Minimising over ordering or stockpiling Immunisation Department, Centre for Infections

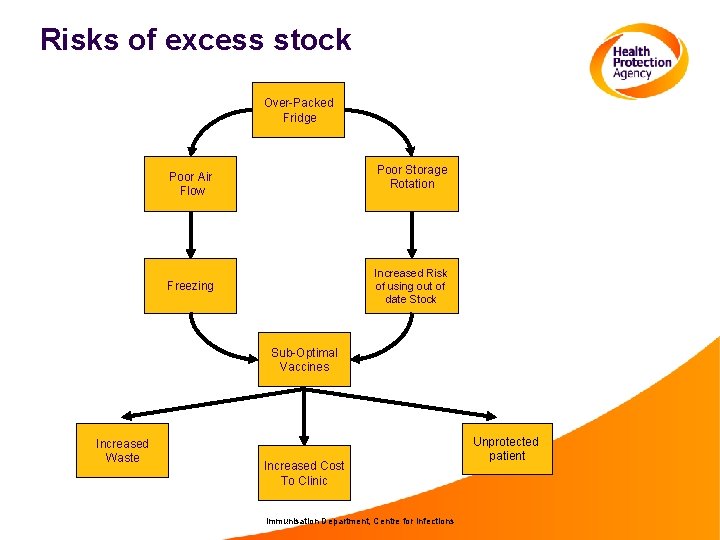
Risks of excess stock Over-Packed Fridge Poor Storage Rotation Poor Air Flow Increased Risk of using out of date Stock Freezing Sub-Optimal Vaccines Increased Waste Increased Cost To Clinic Immunisation Department, Centre for Infections Unprotected patient

Cool Boxes and Transporting vaccines • Use a validated cool box and ice packs from recognised medical supply company • Monitor maximum/minimum temperature, recording at regular intervals • Vaccines should be wrapped in bubble wrap or similar insulation material to prevent direct contact with ice packs • Use insulating material to fill any spaces within the cool box • Only take enough vaccine for particular session and minimise exposure of the vaccines to room temperatures Immunisation Department, Centre for Infections

What to do if there has been a Cold Chain failure Prior to administration ØAny vaccine that has not been stored at a temperature of 2 -8ºC as per its licensing conditions is no longer a licensed product Where there is any doubt that cold chain has not been maintained, vaccines should not be used until further advice has been sought from the vaccine manufacturer ØWritten procedure for the disposal of vaccines by incineration should be available locally Immunisation Department, Centre for Infections

Post administration • Treat as Serious Untoward Incident • Inform Practice Manager/Line Manager/PCT of the incident • Suspend all immunisation clinics until resolved Immunisation Department, Centre for Infections

Best practice in Cold Chain compliance • Defined local policies should be in place and written in accordance with PGDs, SPCs, DH ‘Green Book’ • Regular audit of current practice • Training • Local multidisciplinary support Immunisation Department, Centre for Infections

Useful resources • Chapter 3&4 Green Book: “Storage, distribution and disposal of vaccines” and “Immunisation Procedures” • Poster and plug stickers available to order from DH publications orderline (www. dh. gov. uk) • CDC Vaccine Storage and Handling Toolkit http: //www 2 a. cdc. gov/vaccines/ed/shtoolkit/ • WHO. Temperature sensitivity of vaccines. August 2006. http: //www. who. int/vaccinesdocuments/Docs. PDF 06/847. pdf Immunisation Department, Centre for Infections

Minimum slide set created by: Immunisation Department, Centre for Infections, Health Protection Agency to assist teaching of the Core Curriculum for Immunisation Training (see http: //www. hpa. org. uk/infections/topics_az/vaccination/training_menu. htm) Immunisation Department, Centre for Infections
 Vaccine storage and handling sop worksheet
Vaccine storage and handling sop worksheet Vaccine storage and handling protocol
Vaccine storage and handling protocol Wic vaccine storage
Wic vaccine storage Rabies vaccine storage
Rabies vaccine storage Cold chain short note
Cold chain short note Material handling and storage safety ppt
Material handling and storage safety ppt Emulsion pickoff artifact
Emulsion pickoff artifact Concluding sentence
Concluding sentence What are the steps in narrowing down research topic
What are the steps in narrowing down research topic Secondary storage vs primary storage
Secondary storage vs primary storage Secondary storage vs primary storage
Secondary storage vs primary storage The brittle, rocky outer layer of earth
The brittle, rocky outer layer of earth Mantle earth meaning
Mantle earth meaning Purpose of paradox
Purpose of paradox Uses rigid metallic platters
Uses rigid metallic platters Object based and unified storage
Object based and unified storage Basic layers of the earth
Basic layers of the earth Definition of vaccine
Definition of vaccine Edible vaccine definition
Edible vaccine definition Global vaccine safety initiative gvsi
Global vaccine safety initiative gvsi Anti rabies vaccine dose schedule in india
Anti rabies vaccine dose schedule in india Imocolibov vaccine price
Imocolibov vaccine price Hepatitis b vaccine series adults
Hepatitis b vaccine series adults Varicella zoster complications
Varicella zoster complications