Clinical Asthma Control GINA 2006 No twice or





- Slides: 22


Clinical Asthma Control (GINA 2006) • No (twice or less/week) daytime symptoms • No limitation of daily activities, including exercise • No nocturnal symptoms or awakening because of asthma • No (twice or less/week) need for reliever treatment • Normal or near-normal lung function results • No exacerbations

Asthma management program Component 1. Develop patient/doctor partnership Component 2. Identify and reduce exposure to risk factors Component 3. Assess, treat, and monitor asthma Component 4. Manage asthma exacerbations Component 5. Special Considerations

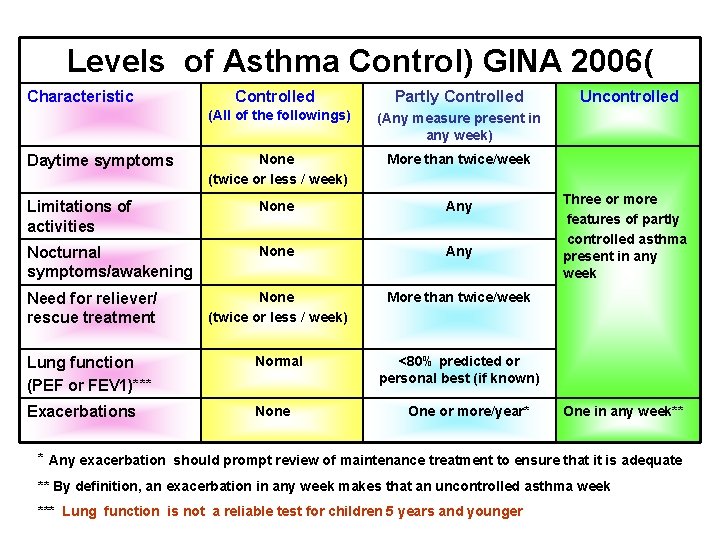
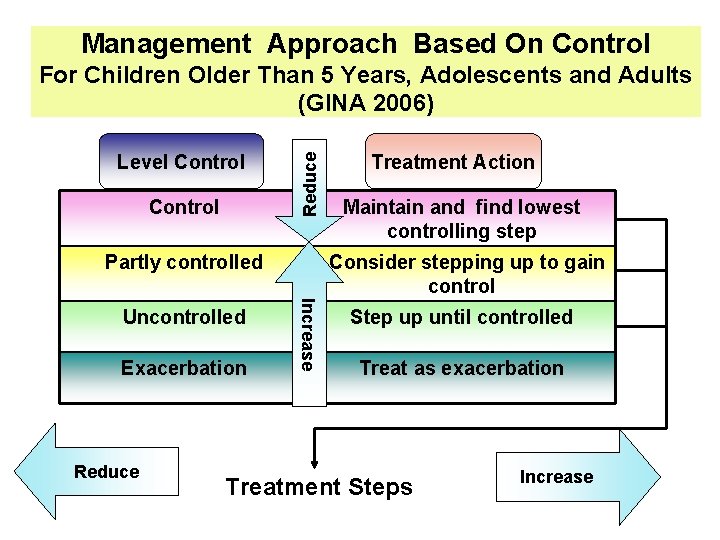
Levels of Asthma Control) GINA 2006( Characteristic Controlled Partly Controlled (All of the followings) (Any measure present in any week) None (twice or less / week) More than twice/week Limitations of activities None Any Nocturnal symptoms/awakening None Any Need for reliever/ rescue treatment None (twice or less / week) More than twice/week Lung function (PEF or FEV 1)*** Normal <80% predicted or personal best (if known) Exacerbations None Daytime symptoms One or more/year* Uncontrolled Three or more features of partly controlled asthma present in any week One in any week** * Any exacerbation should prompt review of maintenance treatment to ensure that it is adequate ** By definition, an exacerbation in any week makes that an uncontrolled asthma week *** Lung function is not a reliable test for children 5 years and younger

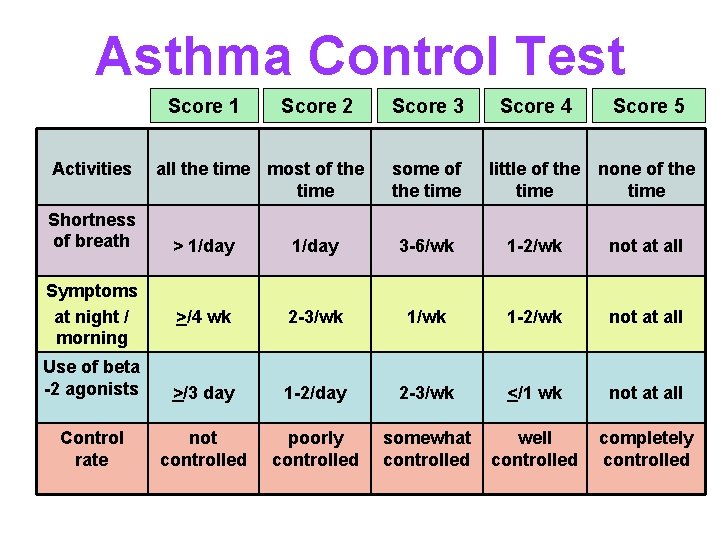
Asthma Control Test Score 1 Activities Shortness of breath Score 2 all the time most of the time Score 3 some of the time Score 4 Score 5 little of the none of the time > 1/day 3 -6/wk 1 -2/wk not at all >/4 wk 2 -3/wk 1 -2/wk not at all Use of beta -2 agonists >/3 day 1 -2/day 2 -3/wk </1 wk not at all Control rate not controlled poorly controlled somewhat controlled well controlled completely controlled Symptoms at night / morning

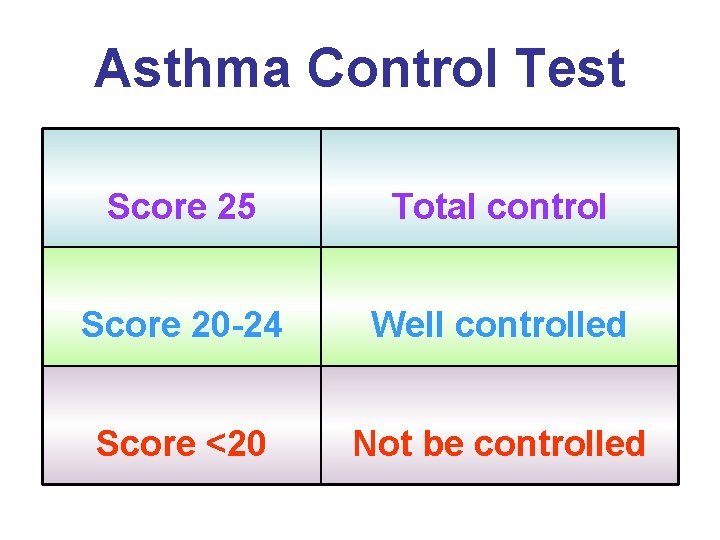
Asthma Control Test Score 25 Total control Score 20 -24 Well controlled Score <20 Not be controlled

Childhood Asthma Control Test Score 0 Score 1 Score 2 Score 3 How is your asthma? Very bad Bad Good Very good Playing / exercises Can’t do Don’t like A little problem No problem Cough All the time Most of the Some of the None of the time Wake up at night All the time Most of the Some of the None of the time

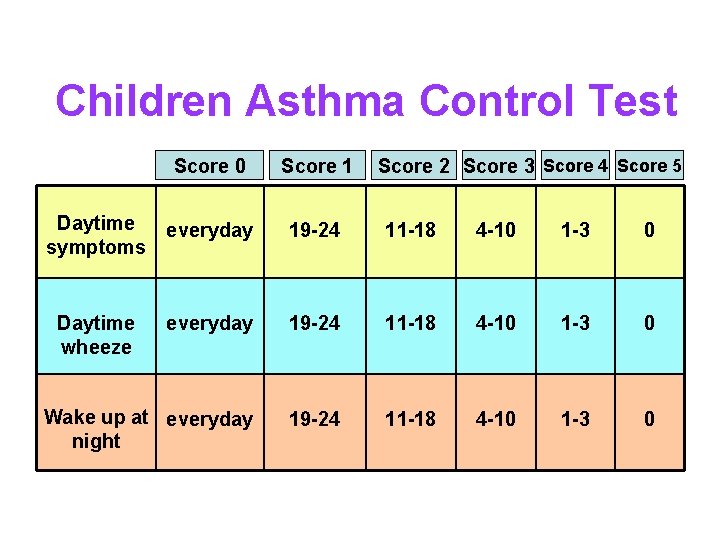
Children Asthma Control Test Score 0 Score 1 Score 2 Score 3 Score 4 Score 5 Daytime symptoms everyday 19 -24 11 -18 4 -10 1 -3 0 Daytime wheeze everyday 19 -24 11 -18 4 -10 1 -3 0 Wake up at everyday night 19 -24 11 -18 4 -10 1 -3 0

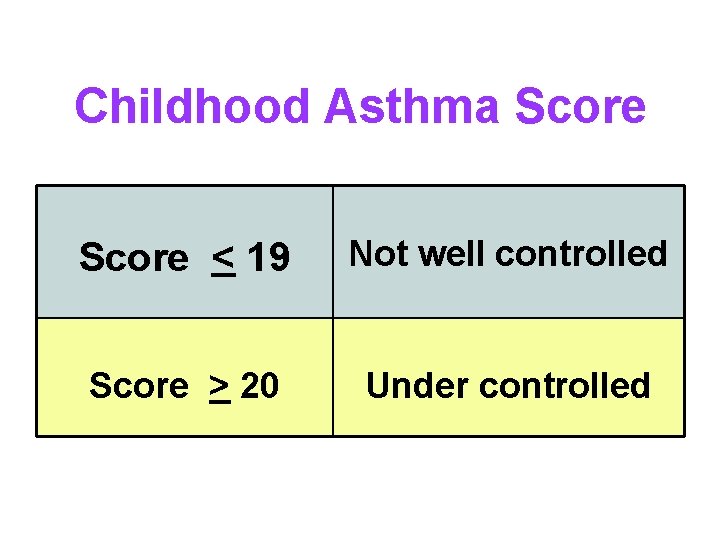
Childhood Asthma Score < 19 Not well controlled Score > 20 Under controlled

Management Approach Based On Control Level Control Reduce For Children Older Than 5 Years, Adolescents and Adults (GINA 2006) Partly controlled Exacerbation Reduce Maintain and find lowest controlling step Consider stepping up to gain control Increase Uncontrolled Treatment Action Step up until controlled Treat as exacerbation Treatment Steps Increase

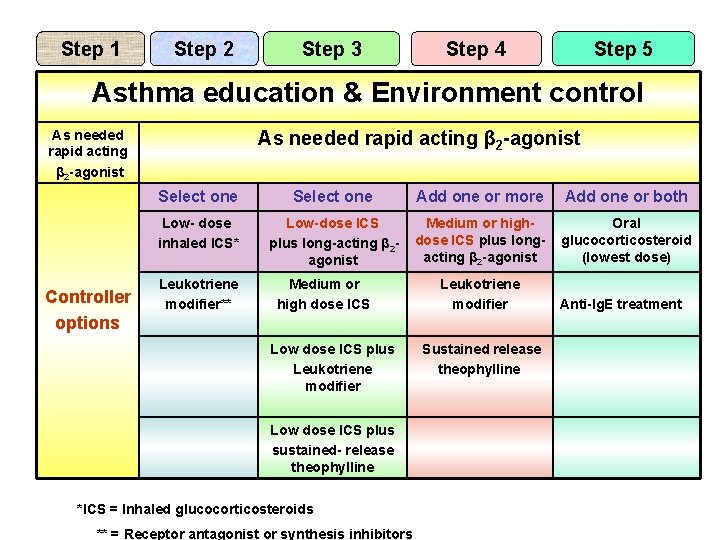
Step 1 Step 2 Step 3 Step 4 Step 5 Asthma education & Environment control As needed rapid acting β 2 -agonist Controller options As needed rapid acting β 2 -agonist Select one Add one or more Add one or both Low- dose inhaled ICS* Low-dose ICS plus long-acting β 2 agonist Medium or highdose ICS plus longacting β 2 -agonist Oral glucocorticosteroid (lowest dose) Leukotriene modifier** Medium or high dose ICS Low dose ICS plus Leukotriene modifier Low dose ICS plus sustained- release theophylline *ICS = Inhaled glucocorticosteroids ** = Receptor antagonist or synthesis inhibitors Leukotriene modifier Sustained release theophylline Anti-Ig. E treatment

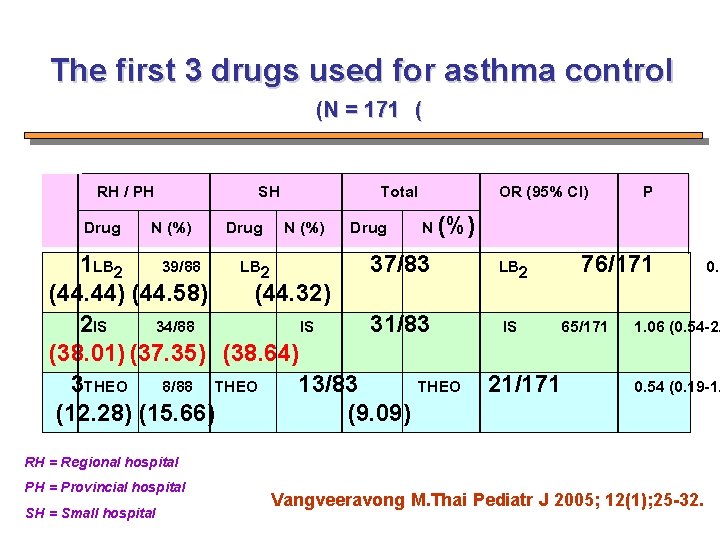
The first 3 drugs used for asthma control (N = 171 ( RH / PH Drug N (%) SH Drug N (%) Total OR (95% CI) Drug N (%) 1 LB 2 39/88 LB 2 37/83 (44. 44) (44. 58) (44. 32) 2 IS 34/88 IS 31/83 (38. 01) (37. 35) (38. 64) 3 THEO 8/88 THEO 13/83 THEO (12. 28) (15. 66) (9. 09) 76/171 LB 2 IS P 65/171 21/171 1. 06 (0. 54 -2. 0. 54 (0. 19 -1. RH = Regional hospital PH = Provincial hospital SH = Small hospital 0. 9 Vangveeravong M. Thai Pediatr J 2005; 12(1); 25 -32.

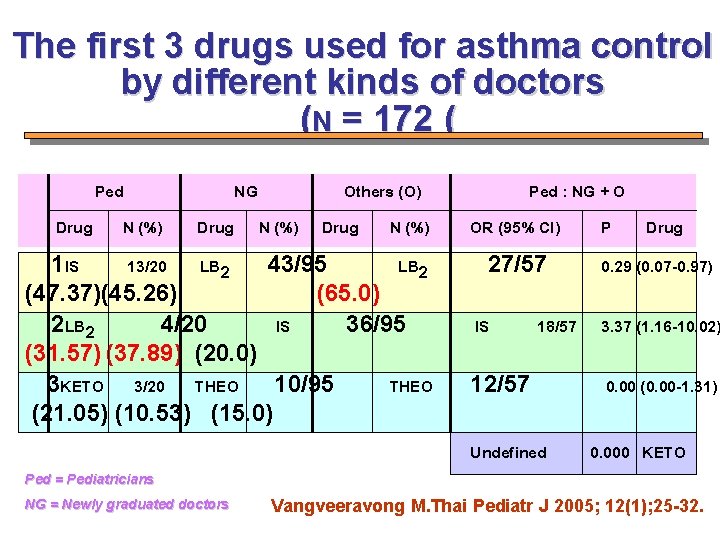
The first 3 drugs used for asthma control by different kinds of doctors (N = 172 ( Ped Drug N (%) NG Drug Others (O) N (%) Drug N (%) 1 IS 13/20 LB 2 43/95 LB 2 (47. 37)(45. 26) (65. 0) 2 LB 2 4/20 IS 36/95 (31. 57) (37. 89) (20. 0) 3 KETO 3/20 THEO 10/95 THEO (21. 05) (10. 53) (15. 0) Ped : NG + O OR (95% CI) P Drug 27/57 IS 18/57 12/57 Undefined 0. 29 (0. 07 -0. 97) 3. 37 (1. 16 -10. 02) 0. 00 (0. 00 -1. 31) 0. 000 KETO Ped = Pediatricians NG = Newly graduated doctors Vangveeravong M. Thai Pediatr J 2005; 12(1); 25 -32.

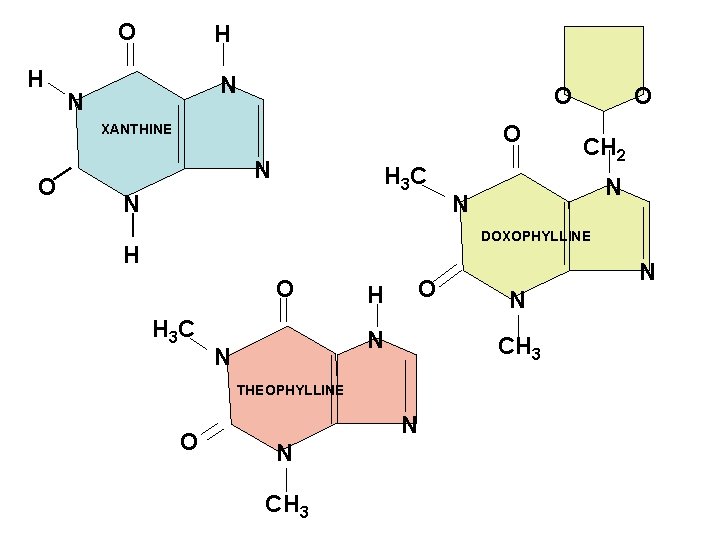
O H H N N O O XANTHINE O N H 3 C N O CH 2 N N DOXOPHYLLINE H O H 3 C H O N N CH 3 THEOPHYLLINE O N

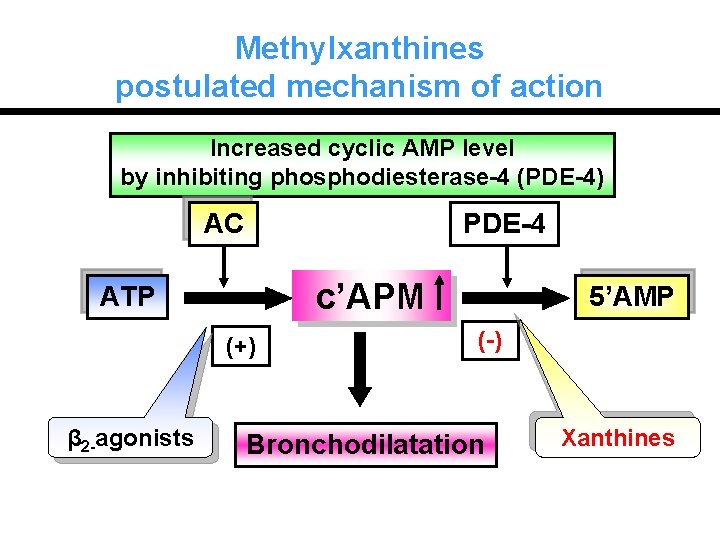
Methylxanthines postulated mechanism of action Increased cyclic AMP level by inhibiting phosphodiesterase-4 (PDE-4) AC PDE-4 c’APM ATP (+) β 2 -agonists 5’AMP (-) Bronchodilatation Xanthines

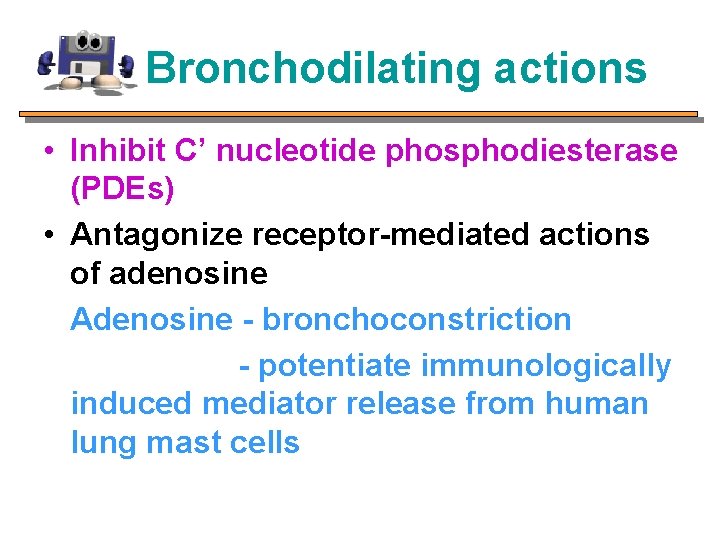
Bronchodilating actions • Inhibit C’ nucleotide phosphodiesterase (PDEs) • Antagonize receptor-mediated actions of adenosine Adenosine - bronchoconstriction - potentiate immunologically induced mediator release from human lung mast cells

Bronchoprotective actions • Reduction of airway responsiveness to “specific” challenges with allergen Hendeles et al. J Allergy Clin Immuno 1995; 95: 505. • Against non-specific stimuli - exercise Pollock et al. Pediatrics 1977; 60: 840. - fog Allegra. Eur J Respir Dis 1980; 61(S), 106: 41. - SO 2 Koenig et al. J Allergy Clin Immunol 1992; 89: 789.

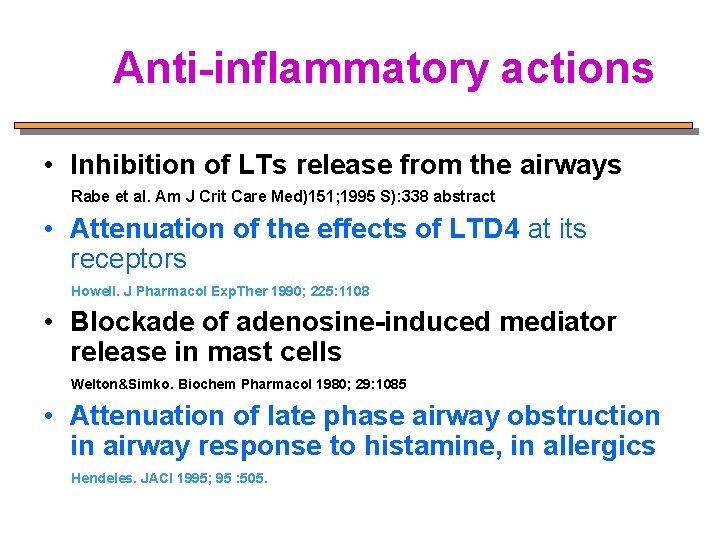
Anti-inflammatory actions • Inhibition of LTs release from the airways Rabe et al. Am J Crit Care Med)151; 1995 S): 338 abstract • Attenuation of the effects of LTD 4 at its receptors Howell. J Pharmacol Exp. Ther 1990; 225: 1108 • Blockade of adenosine-induced mediator release in mast cells Welton&Simko. Biochem Pharmacol 1980; 29: 1085 • Attenuation of late phase airway obstruction in airway response to histamine, in allergics Hendeles. JACI 1995; 95 : 505.

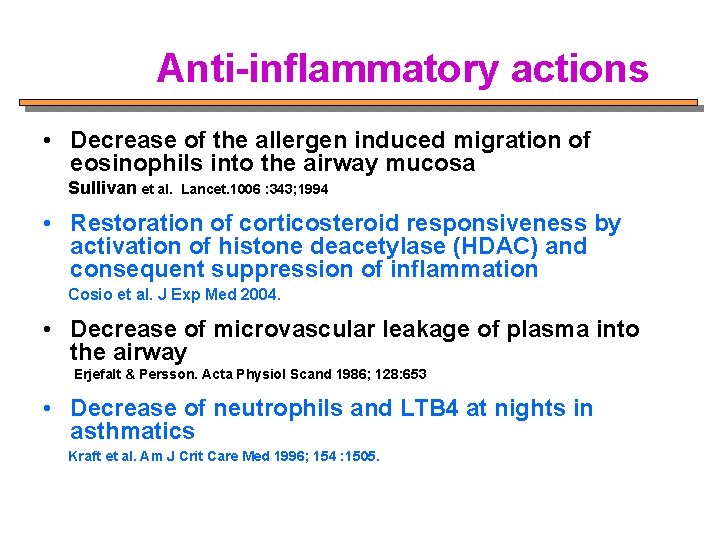
Anti-inflammatory actions • Decrease of the allergen induced migration of eosinophils into the airway mucosa Sullivan et al. Lancet. 1006 : 343; 1994 • Restoration of corticosteroid responsiveness by activation of histone deacetylase (HDAC) and consequent suppression of inflammation Cosio et al. J Exp Med 2004. • Decrease of microvascular leakage of plasma into the airway Erjefalt & Persson. Acta Physiol Scand 1986; 128: 653 • Decrease of neutrophils and LTB 4 at nights in asthmatics Kraft et al. Am J Crit Care Med 1996; 154 : 1505.

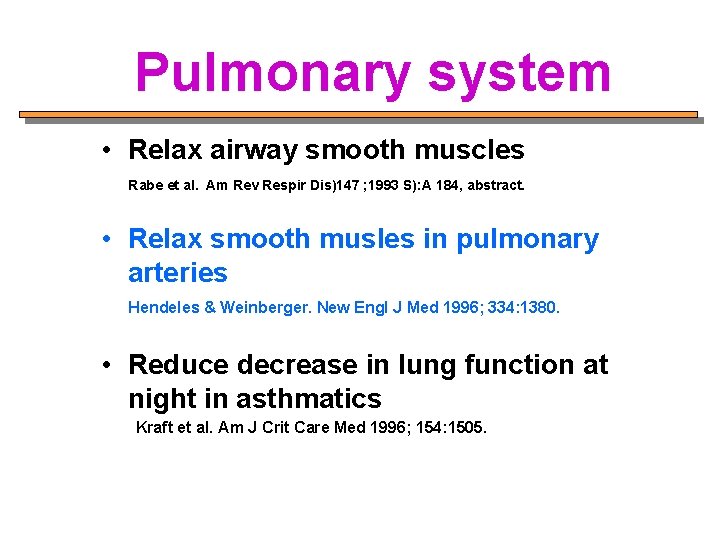
Pulmonary system • Relax airway smooth muscles Rabe et al. Am Rev Respir Dis)147 ; 1993 S): A 184, abstract. • Relax smooth musles in pulmonary arteries Hendeles & Weinberger. New Engl J Med 1996; 334: 1380. • Reduce decrease in lung function at night in asthmatics Kraft et al. Am J Crit Care Med 1996; 154: 1505.

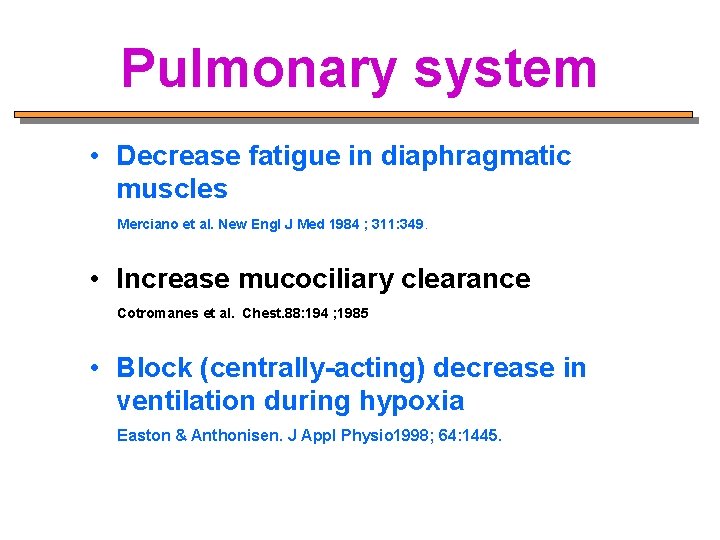
Pulmonary system • Decrease fatigue in diaphragmatic muscles Merciano et al. New Engl J Med 1984 ; 311: 349. • Increase mucociliary clearance Cotromanes et al. Chest. 88: 194 ; 1985 • Block (centrally-acting) decrease in ventilation during hypoxia Easton & Anthonisen. J Appl Physio 1998; 64: 1445.

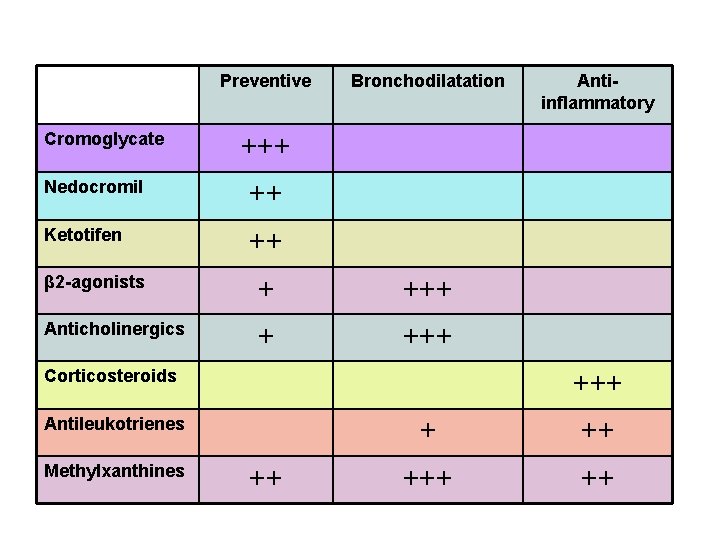
Preventive Cromoglycate Bronchodilatation +++ Nedocromil ++ Ketotifen ++ β 2 -agonists + +++ Anticholinergics + +++ Corticosteroids +++ Antileukotrienes Methylxanthines Antiinflammatory ++ +++ ++
 Gina
Gina Asthma categories
Asthma categories Global initiative for asthma
Global initiative for asthma Copd treatment gold
Copd treatment gold Etiology of bronchial asthma
Etiology of bronchial asthma Gina 2006
Gina 2006 Asthma clinical pathway
Asthma clinical pathway Asthma step up therapy
Asthma step up therapy Gina burkhardt
Gina burkhardt Gina cicco
Gina cicco Gina thorpe
Gina thorpe Gina wilson rational functions
Gina wilson rational functions Global initiative for asthma
Global initiative for asthma Gina's story doncaster
Gina's story doncaster Gina scala
Gina scala Gina salvati
Gina salvati Gina meaning in islam
Gina meaning in islam Photo gina lollobrigida aujourd'hui
Photo gina lollobrigida aujourd'hui Clasificacion de severidad del asma
Clasificacion de severidad del asma Gina fisk
Gina fisk Gina marzano
Gina marzano Gina vanacore
Gina vanacore Gina abbiate
Gina abbiate