Clinical Analytical Chemistry CLS 231 PRACTICAL 3 Precipitation











- Slides: 14

Clinical Analytical Chemistry CLS 231 PRACTICAL (3) Precipitation Titrations Lecturer: Amal Abu - Mostafa 1

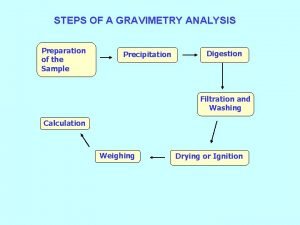
Introduction: In these interactions combine in the solution forming salts less soluble in water For example : interactions of silver nitrate, which is the most important materials used in the interactions of precipitation. Objectives: Calculate the molarity mol/L , and The concentration in g/L of Sodium chloride. 2

Argentometric titration: • Titrations involving silver are termed argentometric, from the Latin name for silver, argentum. Argentometric methods involving precipitation titrimetry: Mohr’s Method Fajan’s Method Volhard’s Method 3

Determination of Chloride Ion Concentration by Titration (Mohr’s Method) Principle: This method determines the chloride ion concentration of a solution by titration with silver nitrate. As the silver nitrate solution is slowly added, a precipitate of silver chloride forms. Ag +(aq) + Cl - (aq) → Ag. Cl(s) 4

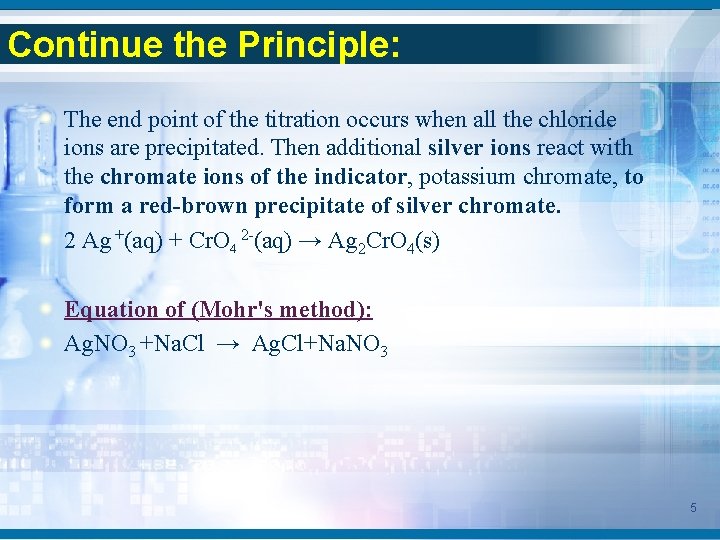
Continue the Principle: The end point of the titration occurs when all the chloride ions are precipitated. Then additional silver ions react with the chromate ions of the indicator, potassium chromate, to form a red-brown precipitate of silver chromate. 2 Ag +(aq) + Cr. O 4 2 -(aq) → Ag 2 Cr. O 4(s) Equation of (Mohr's method): Ag. NO 3 +Na. Cl → Ag. Cl+Na. NO 3 5

Mohr’s method: Note: This method can be used to determine the chloride ion concentration of water samples from many sources such as seawater, stream water, river water and estuary water. 6

Equipments used: burette and stand 10 m. L pipettes. 250 m. L conical flasks Beakers dropper 7

Reagents used: Silver nitrate solution Ag. NO 3(0. 02 mol/L). Sodium chloride Na. Cl solution of Unknown concentration. Potassium chromate K 2 Cr. O 4 solution as an indicator. 8

The Procedure: • Add 10 ml of unknown standard solution of Sodium chloride in conical flask. • Add 4 drops of Potassium Chromate solution • Titrate using (0. 02 M ) Ag. NO 3 silver nitrate solution gradually and slowly from the burette with constant shaking Until the appearance of white precipitate of silver chloride and a red of silver Chromate disappear upon shaking. • Continue to add silver nitrate until the appearance of brick red color • Repeat the experiment twice and then Calculate the concentration of Sodium chloride in mol/L and in g/L. 9

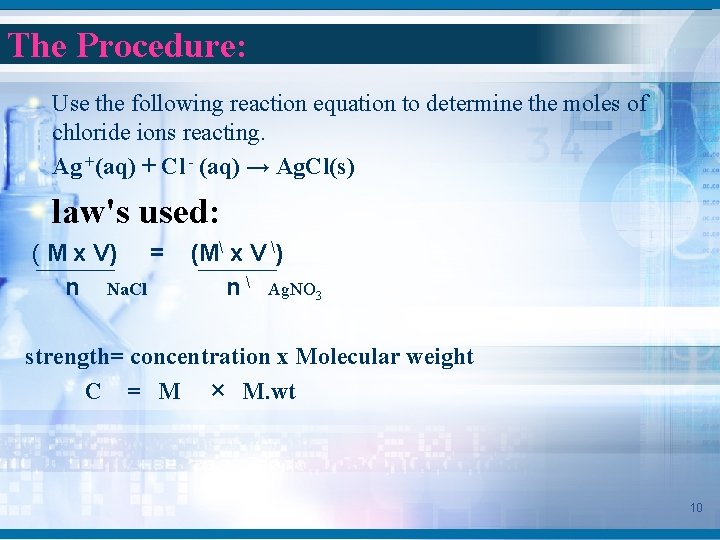
The Procedure: Use the following reaction equation to determine the moles of chloride ions reacting. Ag +(aq) + Cl - (aq) → Ag. Cl(s) law's used: ( M x V) = n Na. Cl (M x V ) n Ag. NO 3 strength= concentration x Molecular weight C = M × M. wt 10

11

Left flask: before the titration endpoint, addition of Ag+ ions leads to formation of silver chloride precipitate, making the solution cloudy. The chromate indicator gives a faint lemon-yellow colour. Centre flask: at the endpoint, all the Cl− ions have precipitated. The slightest excess of Ag+ precipitates with the chromate indicator giving a slight redbrown colouration. Right flask: If addition of Ag+ is continued past the endpoint, further silver chromate precipitate is formed. 12

Notice: This method is used in neutral solutions because in acid solution, silver chromate gets dissolved. And in alkali solution, it makes a precipitate of silver hydroxide. 13

Thank you 14
 Co precipitation and post precipitation
Co precipitation and post precipitation Co precipitation and post precipitation
Co precipitation and post precipitation Normal alt in cats
Normal alt in cats Analytical apparatus
Analytical apparatus Excellence in analytical chemistry
Excellence in analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analysis
Analysis Analytical chemistry chapters
Analytical chemistry chapters Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical measurement range
Analytical measurement range Difference between occlusion and mixed-crystal formation
Difference between occlusion and mixed-crystal formation Example of analytical chemistry
Example of analytical chemistry Tujuan analisis kuantitatif
Tujuan analisis kuantitatif Introduction to analytical chemistry
Introduction to analytical chemistry Normal error curve in analytical chemistry
Normal error curve in analytical chemistry