Classifying Chemical Reactions YEAR 10 CHEMISTRY Physical Chemical


- Slides: 11

Classifying Chemical Reactions YEAR 10 CHEMISTRY

Physical & Chemical Changes What is the difference between a physical and a chemical change? A physical change is one in which no new substance is formed, e. g. ice melting. A chemical change or chemical reaction has occurred if one or more new substances are formed. 2

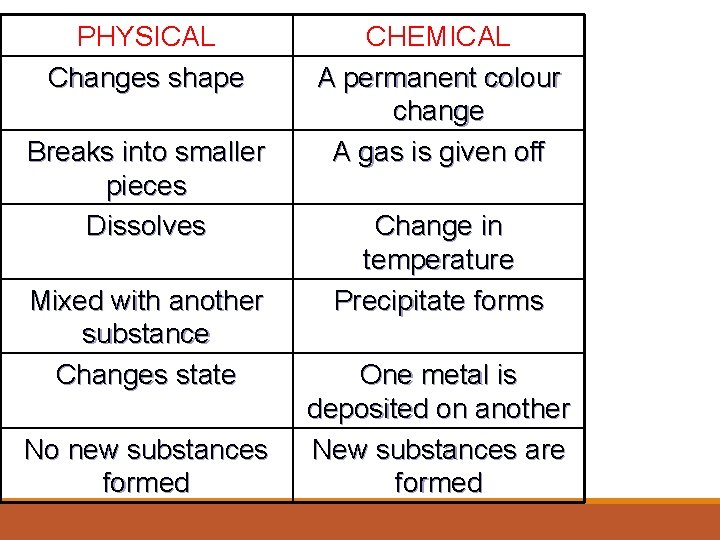
PHYSICAL Changes shape Breaks into smaller pieces Dissolves Mixed with another substance Changes state No new substances formed CHEMICAL A permanent colour change A gas is given off Change in temperature Precipitate forms One metal is deposited on another New substances are formed

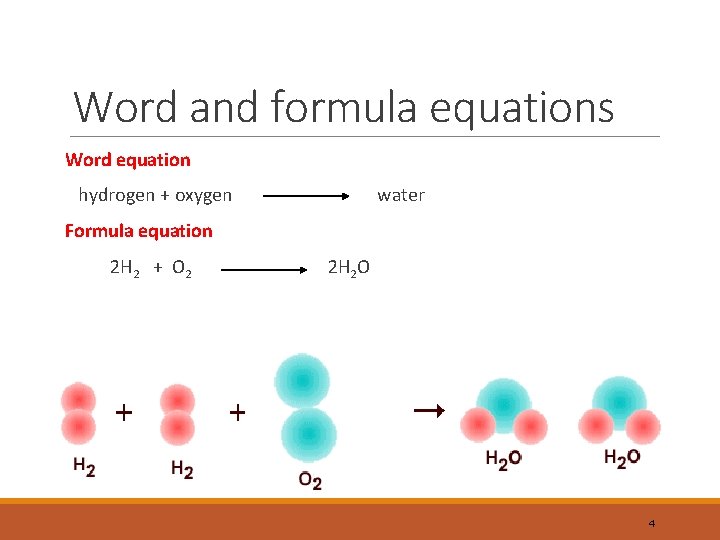
Word and formula equations Word equation hydrogen + oxygen water Formula equation 2 H 2 + O 2 2 H 2 O 4

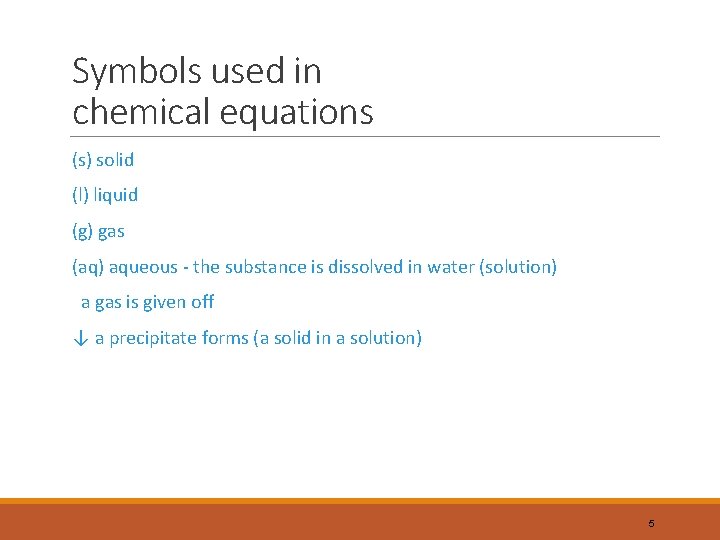
Symbols used in chemical equations (s) solid (l) liquid (g) gas (aq) aqueous - the substance is dissolved in water (solution) a gas is given off ↓ a precipitate forms (a solid in a solution) 5

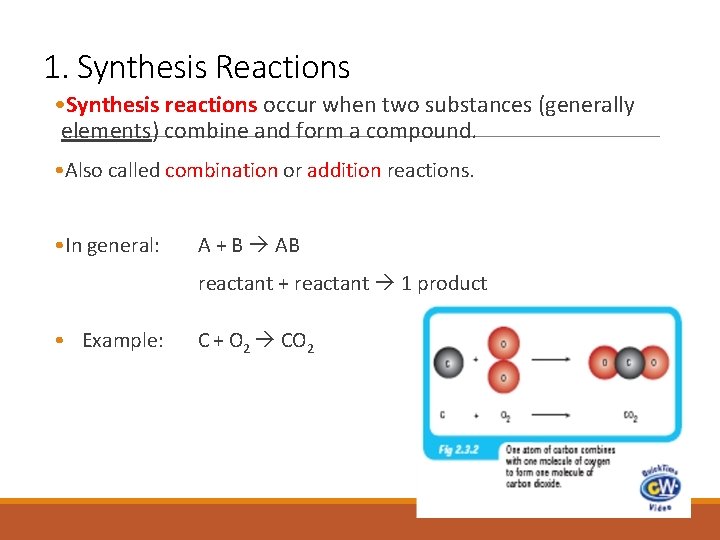
1. Synthesis Reactions • Synthesis reactions occur when two substances (generally elements) combine and form a compound. • Also called combination or addition reactions. • In general: A + B AB reactant + reactant 1 product • Example: C + O 2 CO 2

1. Synthesis Reactions Hydrogen and oxygen yields water 2 H 2 + O 2 2 H 2 O Magnesium plus nitrogen yields magnesium nitride 3 Mg + N 2 Mg 3 N 2 Iron and sulfur yields iron(II) sulfide Fe + S Fe. S 7

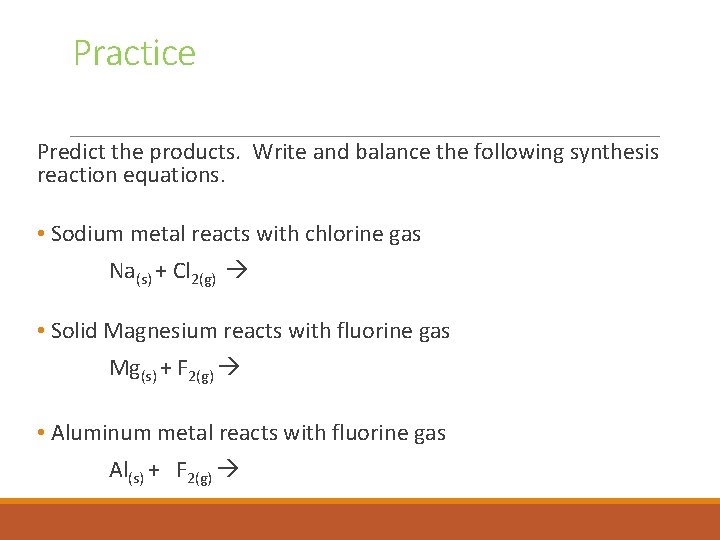
Practice Predict the products. Write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas Na(s) + Cl 2(g) • Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) • Aluminum metal reacts with fluorine gas Al(s) + F 2(g)

2. Decomposition Reactions • Decomposition reactions occur when a compound breaks up into the elements or into a few simpler compounds. • In general: AB A + B 1 Reactant Product + Product • Examples: 2 H 2 O 2 H 2 + O 2 2 Hg. O 2 Hg + O 2

Practice Predict the products. Then write and balance the following decomposition reaction equations: • Solid Lead (IV) oxide decomposes Pb. O 2(s) • Aluminum nitride decomposes Al. N(s)

Practice Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: • N 2(g) + O 2(g) • Ba. CO 3(s) • Co(s)+ S(s) • NH 3(g) + H 2 CO 3(aq)
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal An example of redox reaction
An example of redox reaction Classification and division essay example
Classification and division essay example Types of reactions chemistry
Types of reactions chemistry