Chemistry of hydrogen oxide radicals HOx in Arctic

- Slides: 13

Chemistry of hydrogen oxide radicals (HOx) in Arctic spring Jingqiu Mao, Daniel Jacob Harvard University Jennifer Olson(NASA Langley), Xinrong Ren(U Miami), Bill Brune(Penn State), Paul Wennberg(Caltech), Mike Cubison(U Colorado), Jose L. Jimenez(U Colorado), Ron Cohen(UC Berkeley), Andy Weinheimer(NCAR), Alan Fried(NCAR), Greg Huey (Gatech)

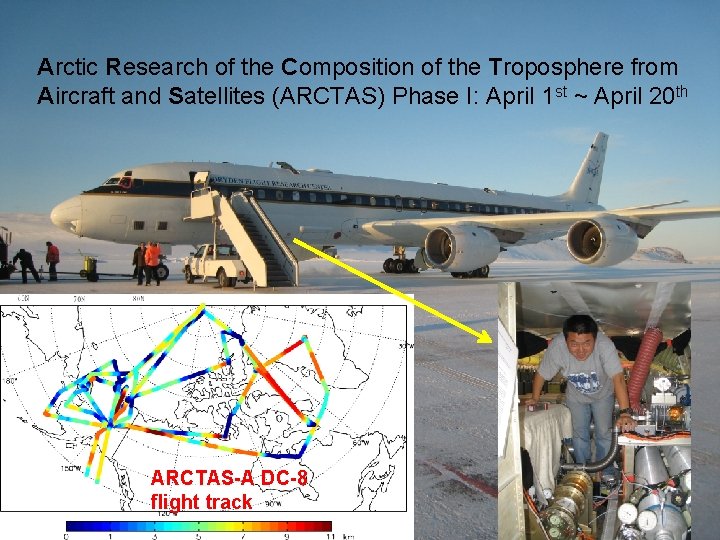
Arctic Research of the Composition of the Troposphere from Aircraft and Satellites (ARCTAS) Phase I: April 1 st ~ April 20 th ARCTAS-A DC-8 flight track

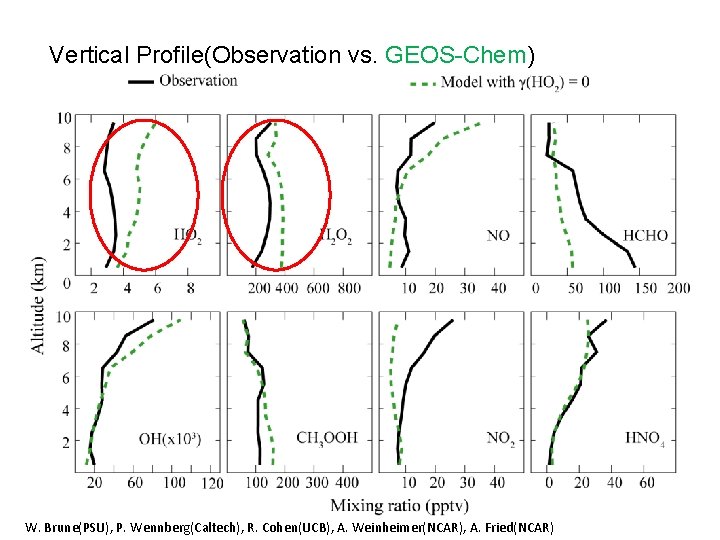
Vertical Profile(Observation vs. GEOS-Chem) W. Brune(PSU), P. Wennberg(Caltech), R. Cohen(UCB), A. Weinheimer(NCAR), A. Fried(NCAR)

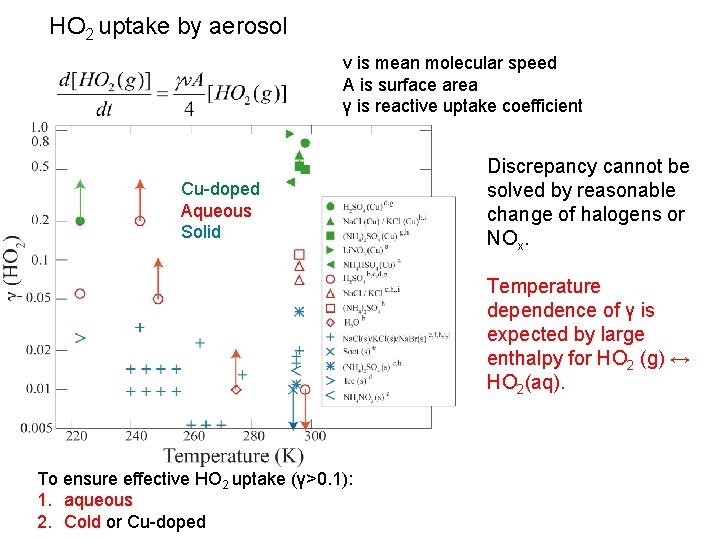
HO 2 uptake by aerosol ν is mean molecular speed A is surface area γ is reactive uptake coefficient Cu-doped Aqueous Solid Discrepancy cannot be solved by reasonable change of halogens or NOx. Temperature dependence of γ is expected by large enthalpy for HO 2 (g) ↔ HO 2(aq). To ensure effective HO 2 uptake (γ>0. 1): 1. aqueous 2. Cold or Cu-doped

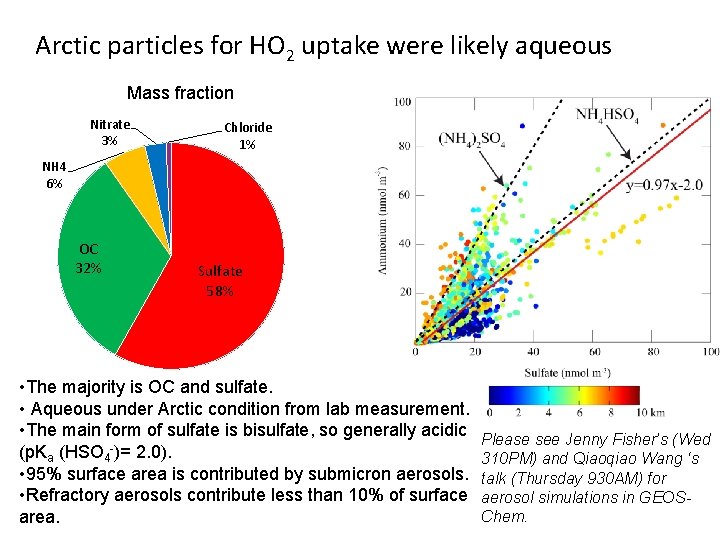
Arctic particles for HO 2 uptake were likely aqueous Mass fraction Nitrate 3% Chloride 1% NH 4 6% OC 32% Sulfate 58% • The majority is OC and sulfate. • Aqueous under Arctic condition from lab measurement. • The main form of sulfate is bisulfate, so generally acidic (p. Ka (HSO 4 -)= 2. 0). • 95% surface area is contributed by submicron aerosols. • Refractory aerosols contribute less than 10% of surface area. Please see Jenny Fisher’s (Wed 310 PM) and Qiaoqiao Wang ‘s talk (Thursday 930 AM) for aerosol simulations in GEOSChem.

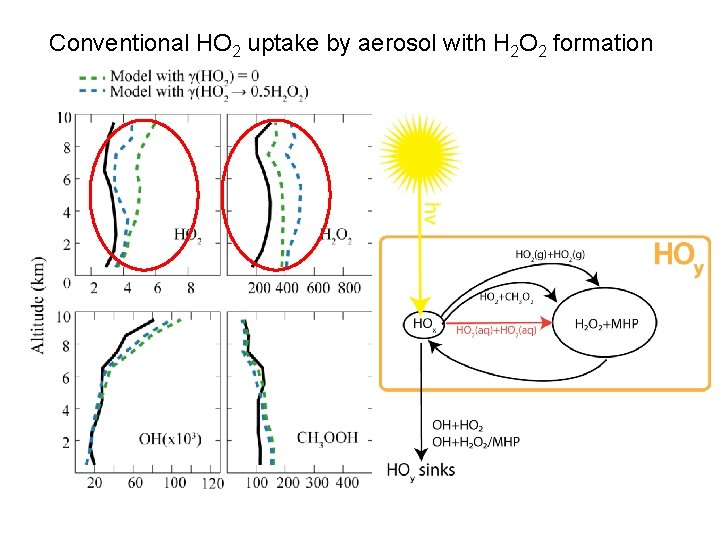
Conventional HO 2 uptake by aerosol with H 2 O 2 formation

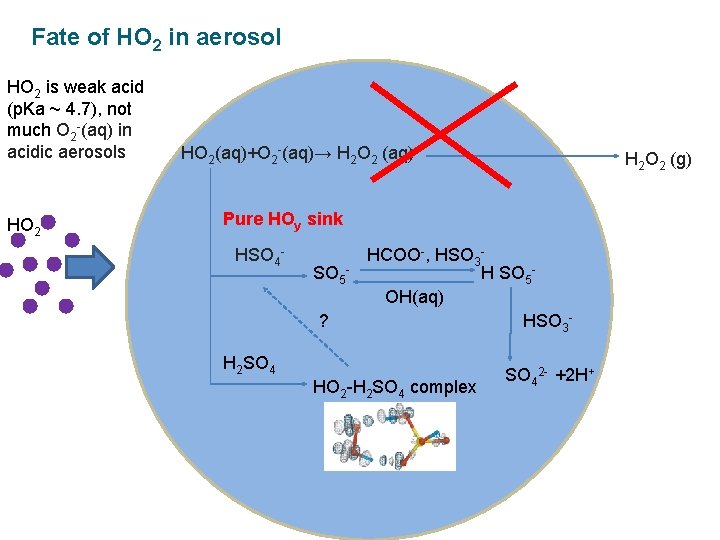
Fate of HO 2 in aerosol HO 2 is weak acid (p. Ka ~ 4. 7), not much O 2 -(aq) in acidic aerosols HO 2(aq)+O 2 -(aq)→ H 2 O 2 (aq) H 2 O 2 (g) Pure HOy sink HSO 4 - SO 5? HCOO-, HSO 3 H SO 5 OH(aq) HSO 3 - H 2 SO 4 HO 2 -H 2 SO 4 complex SO 42 - +2 H+

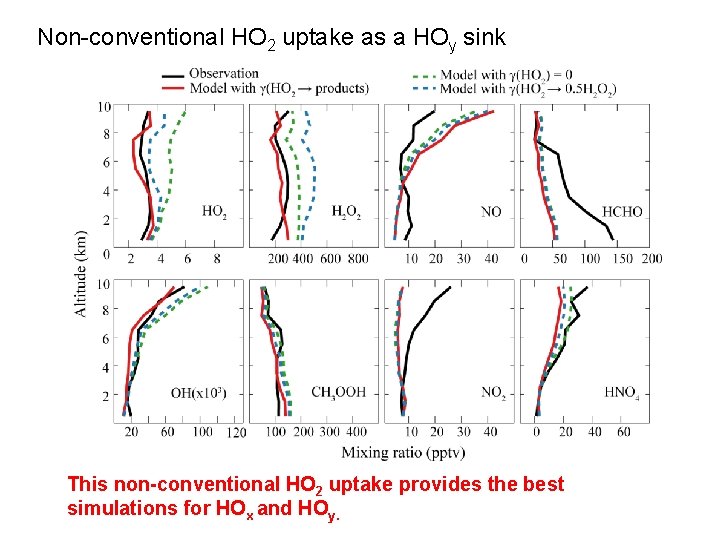
Non-conventional HO 2 uptake as a HOy sink This non-conventional HO 2 uptake provides the best simulations for HOx and HOy.

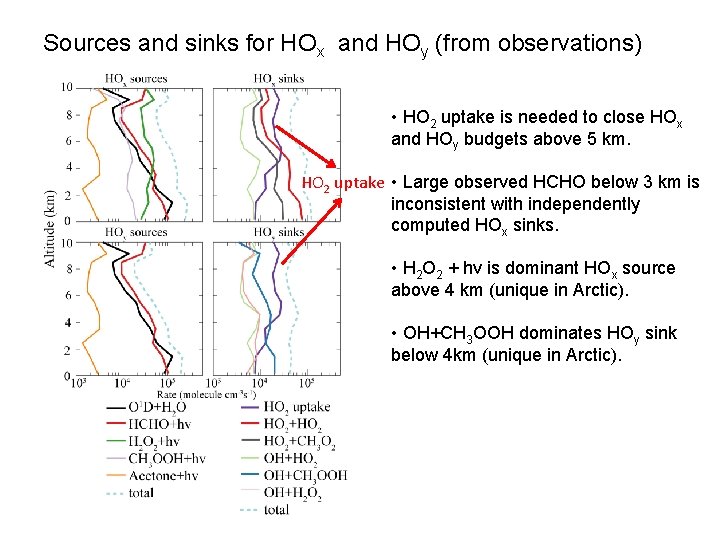
Sources and sinks for HOx and HOy (from observations) • HO 2 uptake is needed to close HOx and HOy budgets above 5 km. HO 2 uptake • Large observed HCHO below 3 km is inconsistent with independently computed HOx sinks. • H 2 O 2 + hv is dominant HOx source above 4 km (unique in Arctic). • OH+CH 3 OOH dominates HOy sink below 4 km (unique in Arctic).

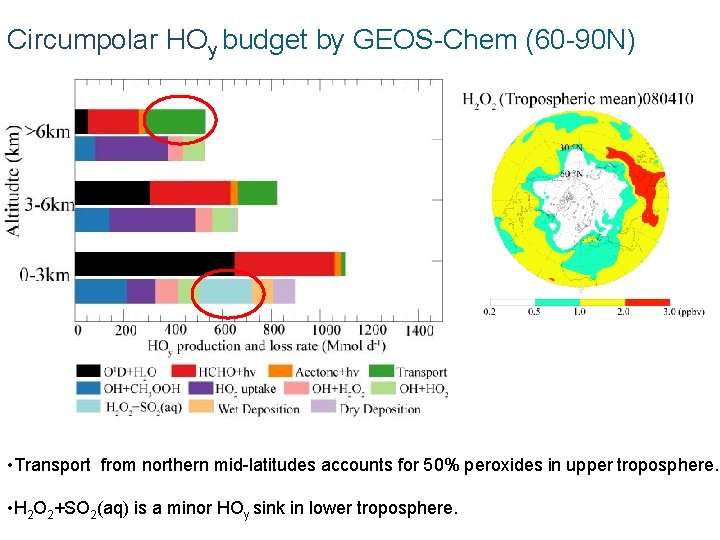
Circumpolar HOy budget by GEOS-Chem (60 -90 N) • Transport from northern mid-latitudes accounts for 50% peroxides in upper troposphere. • H 2 O 2+SO 2(aq) is a minor HOy sink in lower troposphere.

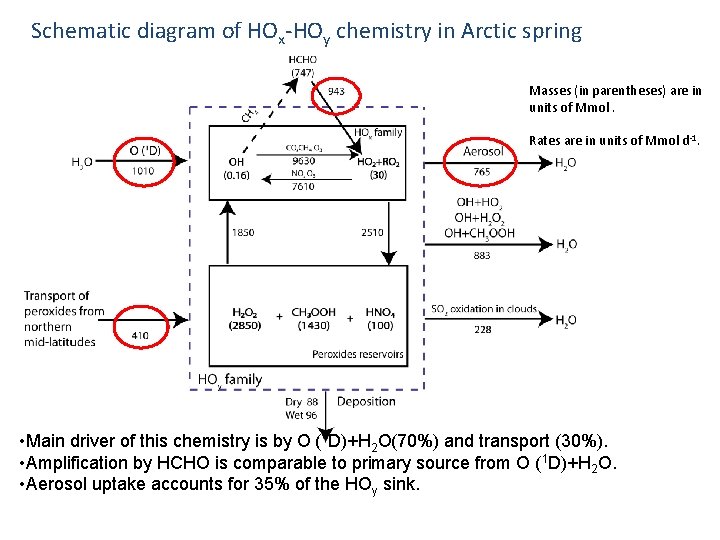
Schematic diagram of HOx-HOy chemistry in Arctic spring Masses (in parentheses) are in units of Mmol. Rates are in units of Mmol d-1. • Main driver of this chemistry is by O (1 D)+H 2 O(70%) and transport (30%). • Amplification by HCHO is comparable to primary source from O (1 D)+H 2 O. • Aerosol uptake accounts for 35% of the HOy sink.

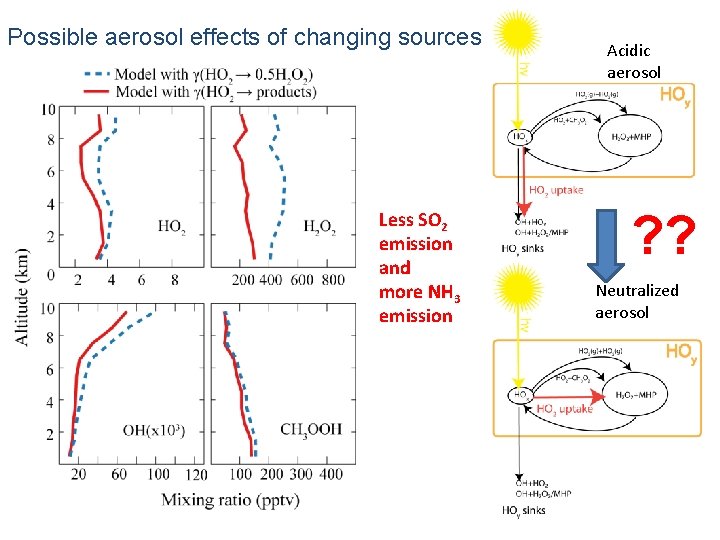
Possible aerosol effects of changing sources Less SO 2 emission and more NH 3 emission Acidic aerosol ? ? Neutralized aerosol

Conclusions Cold temperature, high aerosol loading and slow photochemical cycling suggest the important role of HO 2 uptake in HOx chemistry in Arctic spring. With HO 2 uptake as a HOy sink, we successfully reproduce HOx and their reservoirs in the model. HO 2 uptake accounts for 35% of HOy sink. Successful simulation of observed HO 2 and H 2 O 2 in ARCTAS implies HO 2 uptake that does not produce H 2 O 2 – possible mechanism coupled to HSO 4/H 2 SO 4 producing HSO 5 -. The photolysis of H 2 O 2 becomes the dominant HOx source in middle and upper troposphere due to the long lifetime of HOy combined with the efficient cycling between HOx radicals and peroxide reservoirs. Future changes in aerosol acidity due to decreasing SO 2 and increasing NH 3 could lead to different chemistry and possibly increase OH.