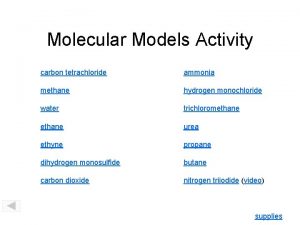
Chemistry of Hydrogen Chemistry of Hydrogen 1 H













































- Slides: 94

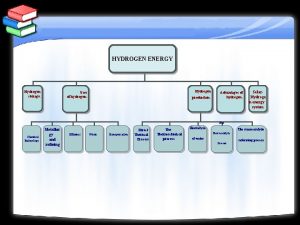
Chemistry of Hydrogen

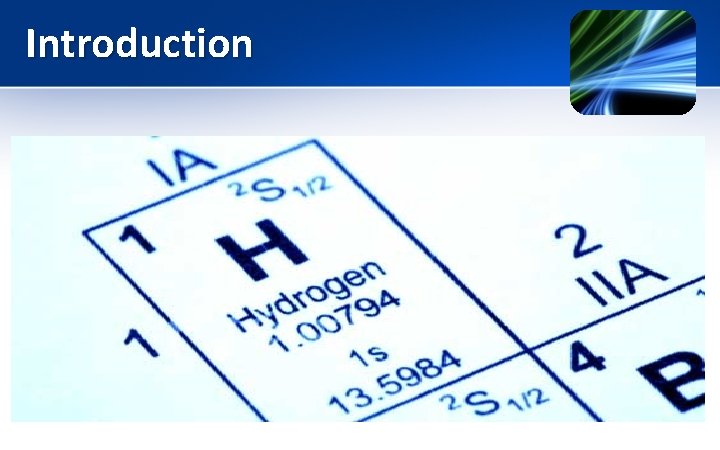
Chemistry of Hydrogen (1 H) • The first element of the periodic table. • Atomic Number = 1 • Atomic weight = 1. 0079 • Electronic configuration = 1 s 1

Introduction

Isotopes of Hydrogen 1. Protium / Hydrogen 2. Deuterium 3. Tritium

Isotopes of Hydrogen 1) Protium / Hydrogen (H) § It is the most commonly available isotope. § It constitutes 99% of total hydrogen available in nature. § The molecule of ordinary hydrogen is diatomic (H 2) § The nucleus of atom consist of single proton & no neutron (mass number = 1). § It is represented by .

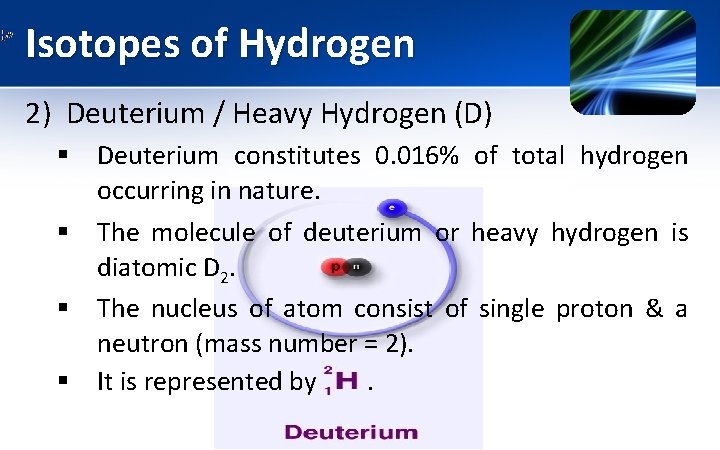
Isotopes of Hydrogen 2) Deuterium / Heavy Hydrogen (D) § Deuterium constitutes 0. 016% of total hydrogen occurring in nature. § The molecule of deuterium or heavy hydrogen is diatomic D 2. § The nucleus of atom consist of single proton & a neutron (mass number = 2). § It is represented by .

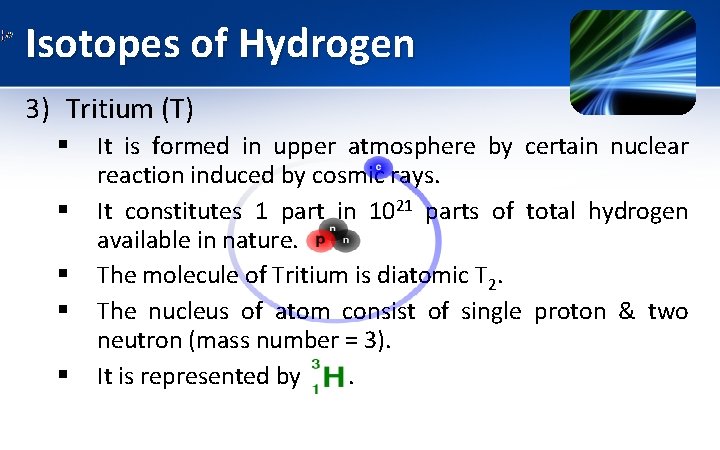
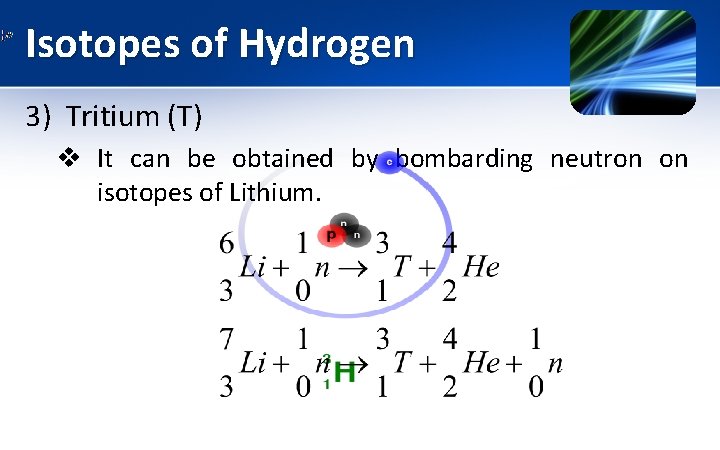
Isotopes of Hydrogen 3) Tritium (T) § § § It is formed in upper atmosphere by certain nuclear reaction induced by cosmic rays. It constitutes 1 part in 1021 parts of total hydrogen available in nature. The molecule of Tritium is diatomic T 2. The nucleus of atom consist of single proton & two neutron (mass number = 3). It is represented by .

Isotopes of Hydrogen 3) Tritium (T) v It is radioactive in nature. v Tritium decays by the loss of β particle to yield rare but stable isotope of helium.

Isotopes of Hydrogen 3) Tritium (T) v It can be obtained by bombarding neutron on isotopes of Lithium.

Importance/ Applications of Isotopes 1) Use of deuterium & tritium in nuclear energy § In fusion reactor, tritium & deuterium are heated to give a plasma in which the nuclei react to produce a neutron & . § Energy obtained per unit mass of deuterium & tritium nuclei is about 4 times more than that from fission of Uranium & 10 million times more than from petrol.

Nuclear Reaction of Deuterium & Tritium

Importance of Isotopes 2) Heavy water (D 2 O): • used as neutron moderator & Coolant for nuclear reactors • Decides reaction mechanism • For synthesis of organic compounds used as solvents in NMR spectroscopy

Importance of Isotopes 3) Kinetic Isotope effect: § Differences in the properties which arise from the difference in mass are called as isotope effect. § Rates of reactions are measurable different for the process. § The detection of this kinetic isotope effect help to support a proposed reaction mechanism of many chemical reactions.

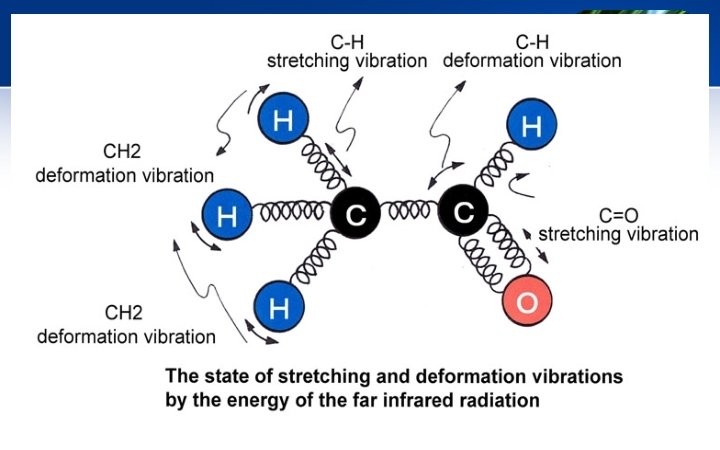
Importance of Isotopes 4) Isotope effect in detection of motion of hydrogen: § The heavier isotope (D) results in lower frequency. § This isotope effect can be studied by IR spectra of H & D substituted molecule to determine motion of H atom in the molecule.

Importance of Isotopes 5) Isotopes as tracers: § The distinct properties of isotopes makes them useful as tracers. § H & D in various reactions by IR & mass spectroscopy. § Tritium can be detected by its radioactivity.


Importance of Isotopes 6) Use in NMR (Nuclear Magnetic Resonance) Spectroscopy: § 1 H-NMR detects the presence of hydrogen nuclei in compound & is powerful method for structure determination of molecule, even like protein.

Importance of Isotopes 7) Tritium in self powered lighting devices. § Tritium is used in specialized self powered lighting devices. § The emitted electrons from radioactive decay of small amount of tritium cause phosphors (A phosphor, most generally, is a substance that exhibits the phenomenon of luminescence) to glow.

Importance of Isotopes 8) Tritium in nuclear weapon: § Tritium is used as nuclear weapons to enhance efficiency & yield of fission bombs. § It is used in hydrogen bomb.

Methods of Preparation 1. Laboratory Scale Preparation 2. Industrial Production 3. From Solar Energy

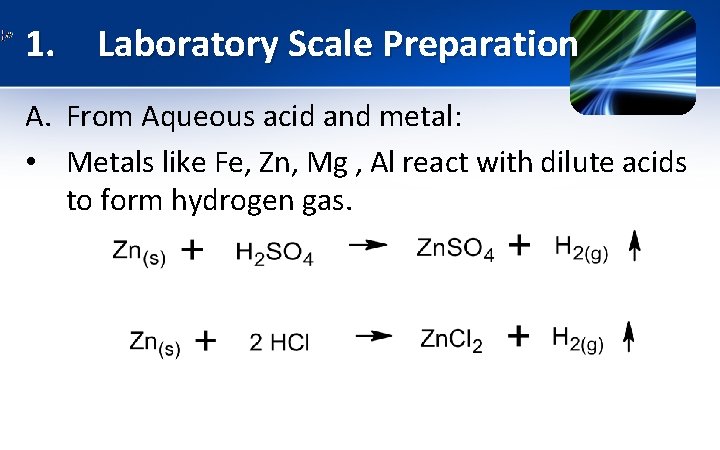
1. Laboratory Scale Preparation A. From Aqueous acid and metal: • Metals like Fe, Zn, Mg , Al react with dilute acids to form hydrogen gas.

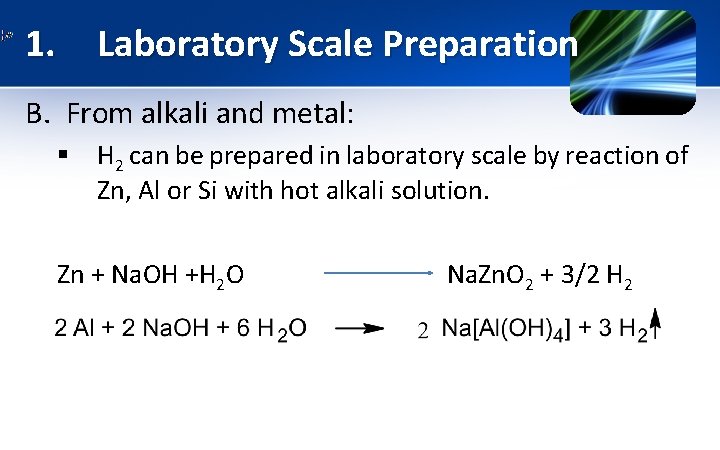
1. Laboratory Scale Preparation B. From alkali and metal: § H 2 can be prepared in laboratory scale by reaction of Zn, Al or Si with hot alkali solution. Zn + Na. OH +H 2 O Na. Zn. O 2 + 3/2 H 2

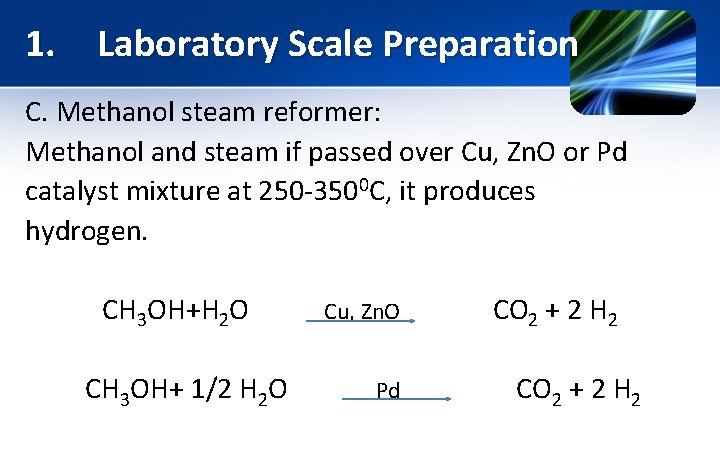
1. Laboratory Scale Preparation C. Methanol steam reformer: Methanol and steam if passed over Cu, Zn. O or Pd catalyst mixture at 250 -3500 C, it produces hydrogen. CH 3 OH+H 2 O Cu, Zn. O CO 2 + 2 H 2 CH 3 OH+ 1/2 H 2 O Pd CO 2 + 2 H 2

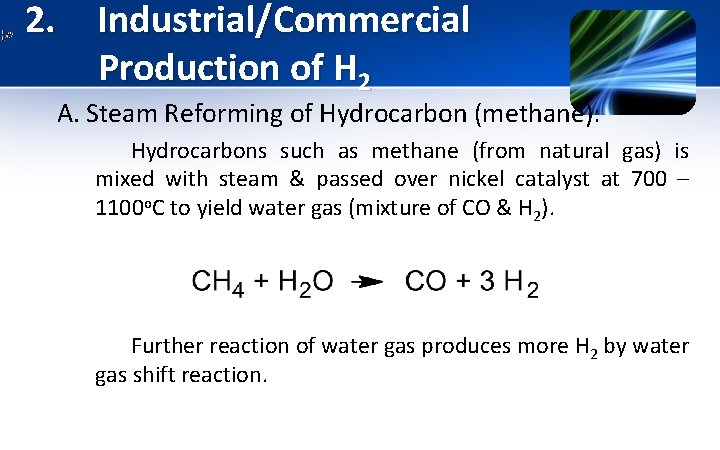
2. Industrial/Commercial Production of H 2 A. Steam Reforming of Hydrocarbon (methane): Hydrocarbons such as methane (from natural gas) is mixed with steam & passed over nickel catalyst at 700 – 1100 o. C to yield water gas (mixture of CO & H 2). Further reaction of water gas produces more H 2 by water gas shift reaction.

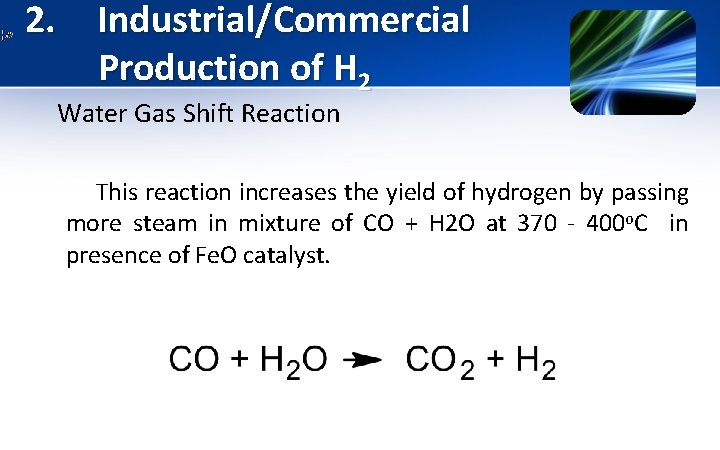
2. Industrial/Commercial Production of H 2 Water Gas Shift Reaction This reaction increases the yield of hydrogen by passing more steam in mixture of CO + H 2 O at 370 - 400 o. C in presence of Fe. O catalyst.

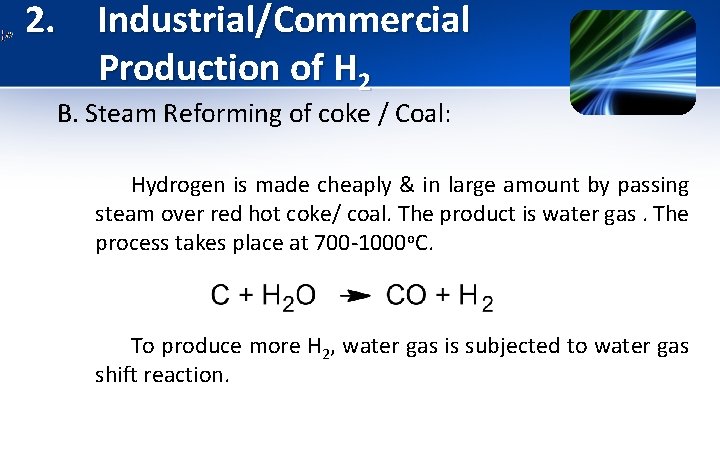
2. Industrial/Commercial Production of H 2 B. Steam Reforming of coke / Coal: Hydrogen is made cheaply & in large amount by passing steam over red hot coke/ coal. The product is water gas. The process takes place at 700 -1000 o. C. To produce more H 2, water gas is subjected to water gas shift reaction.

2. Industrial/Commercial Production of H 2 Water Gas Shift Reaction This reaction increases the yield of hydrogen by passing more steam in mixture of CO + H 2 O at 370 - 400 o. C in presence of Fe. O catalyst.

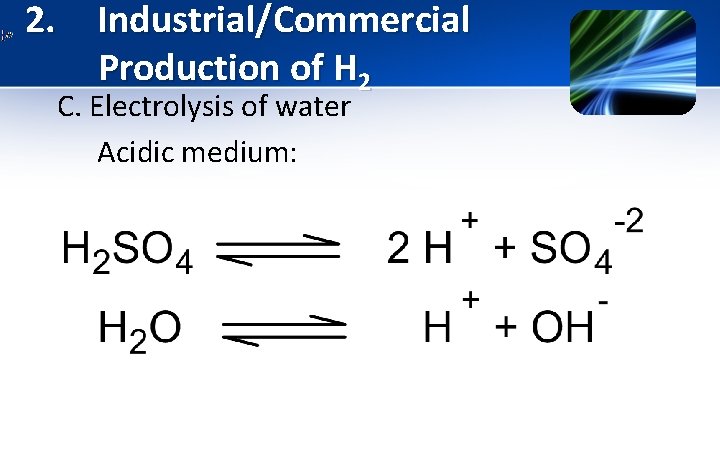
2. Industrial/Commercial Production of H 2 C. Electrolysis of water Acidic medium:

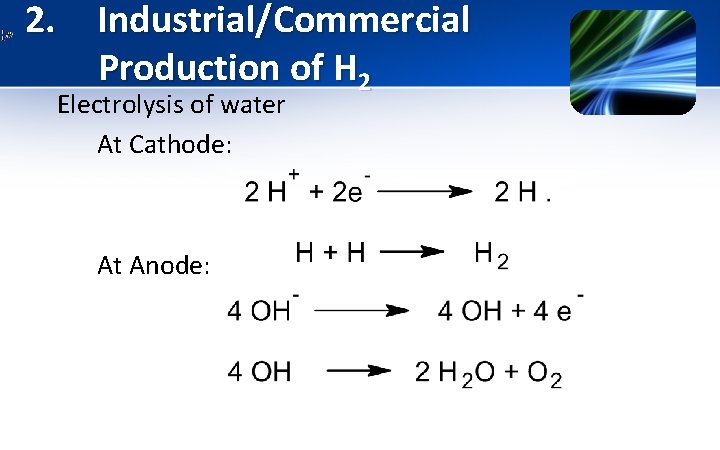
2. Industrial/Commercial Production of H 2 Electrolysis of water At Cathode: At Anode:

3. From Solar Energy: A. Water splitting (thermal Process) § Water splitting is the general term for a chemical reaction in which water is separated into oxygen & hydrogen by solar heat.

Ø Reactions involved • In presence of metal oxides at about 2200 o. C, water splits into hydrogen and oxygen. • Hydrogen needs to be sepataed from the mixture

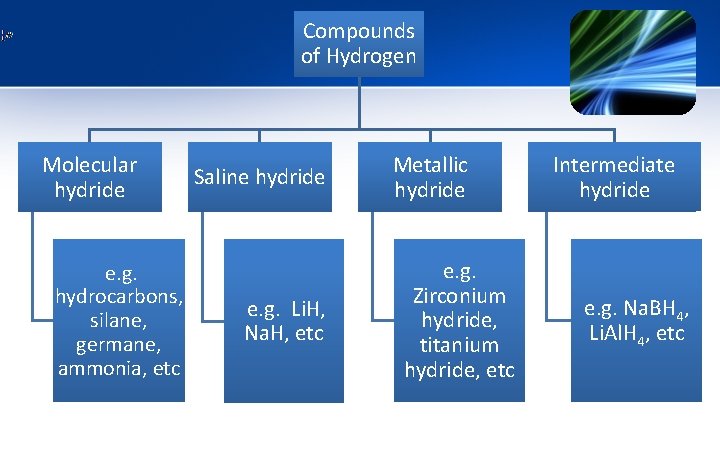
Compounds of Hydrogen 1. Molecular hydrides 2. Saline hydrides 3. Metallic hydrides 4. Intermediate hydride

Compounds of Hydrogen Molecular hydride e. g. hydrocarbons, silane, germane, ammonia, etc Saline hydride e. g. Li. H, Na. H, etc Metallic hydride e. g. Zirconium hydride, titanium hydride, etc Intermediate hydride e. g. Na. BH 4, Li. Al. H 4, etc

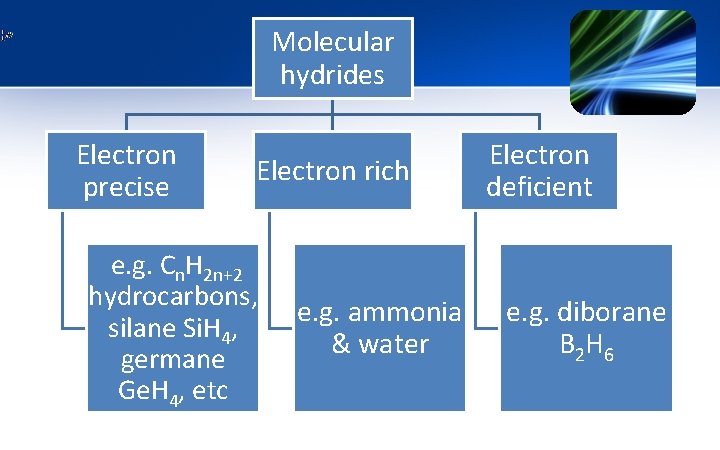
Molecular hydrides Electron precise Electron rich e. g. Cn. H 2 n+2 hydrocarbons, e. g. ammonia silane Si. H 4, & water germane Ge. H 4, etc Electron deficient e. g. diborane B 2 H 6

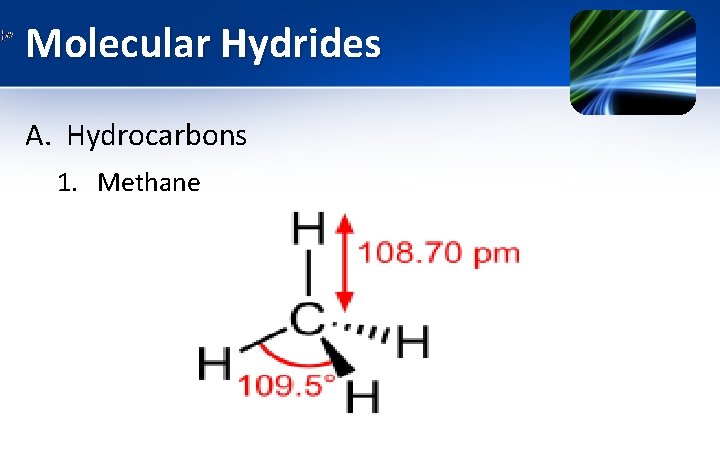
Molecular Hydrides A. Hydrocarbons 1. Methane

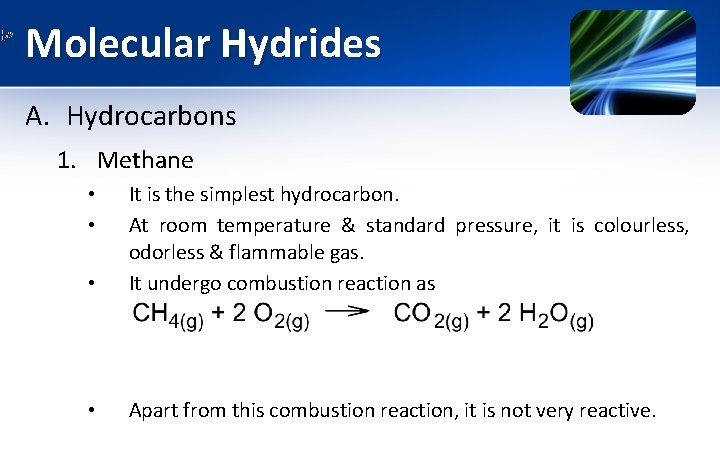
Molecular Hydrides A. Hydrocarbons 1. Methane • It is the simplest hydrocarbon. At room temperature & standard pressure, it is colourless, odorless & flammable gas. It undergo combustion reaction as • Apart from this combustion reaction, it is not very reactive. • •

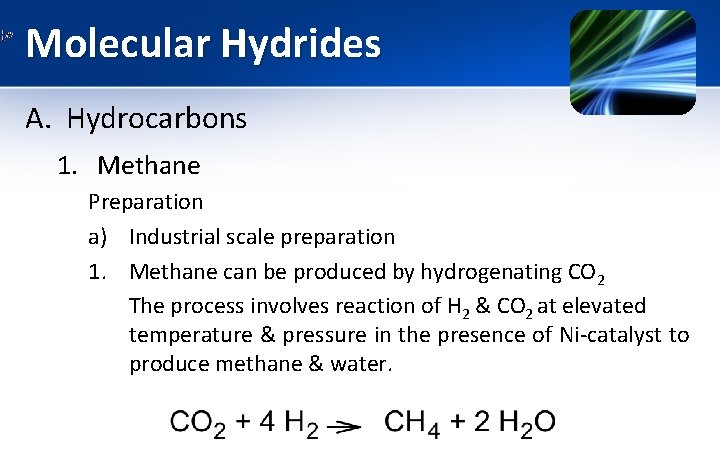
Molecular Hydrides A. Hydrocarbons 1. Methane Preparation a) Industrial scale preparation 1. Methane can be produced by hydrogenating CO 2 The process involves reaction of H 2 & CO 2 at elevated temperature & pressure in the presence of Ni-catalyst to produce methane & water.

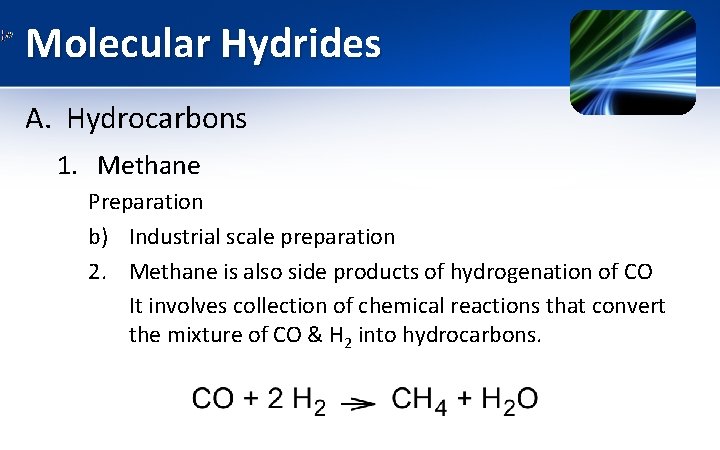
Molecular Hydrides A. Hydrocarbons 1. Methane Preparation b) Industrial scale preparation 2. Methane is also side products of hydrogenation of CO It involves collection of chemical reactions that convert the mixture of CO & H 2 into hydrocarbons.

Molecular Hydrides A. Hydrocarbons 1. Methane Applications a) It is used as domestic & industrial fuel. Methane in the form of compressed natural gas is used as vehicular fuel. It is a clean burning fuel b) It is important for electrical generation by burning it as a fuel in a gas turbine or steam engine. c) Chemical feedstock – in chemical industries, methane is converted to synthesis gas, a mixture of CO & H 2, by steam reforming.

Molecular Hydrides A. Hydrocarbons 1. Ethane

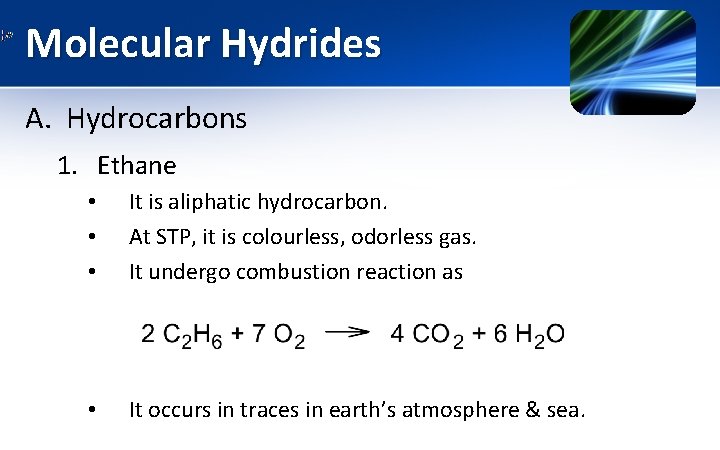
Molecular Hydrides A. Hydrocarbons 1. Ethane • • • It is aliphatic hydrocarbon. At STP, it is colourless, odorless gas. It undergo combustion reaction as • It occurs in traces in earth’s atmosphere & sea.

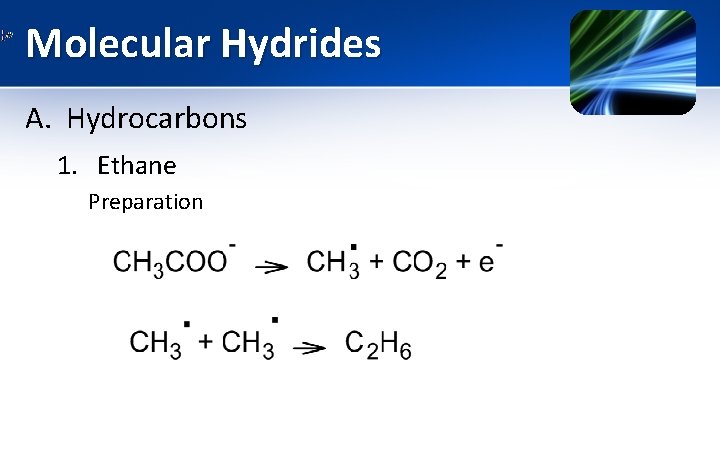
Molecular Hydrides A. Hydrocarbons 1. Ethane Preparation a) Laboratory scale preparation • Ethane can be prepared by electrolysis, In this technique an aqueous solution of acetate salt is electrolyzed. • At anode acetate is oxidized to produce CO 2 & methyl radical & highly reactive methyl radicals combine to produce ethane.

Molecular Hydrides A. Hydrocarbons 1. Ethane Preparation

Molecular Hydrides A. Hydrocarbons 1. Ethane Applications a) It is mainly used in chemical industries in the production of ethylene. It is a raw material for polymer formation. b) It can be used as a refrigerant in cryogenic refrigeration system. c) In scientific research, liquid ethane is used in cryoelectron microscopy.

Molecular Hydrides B. Silane

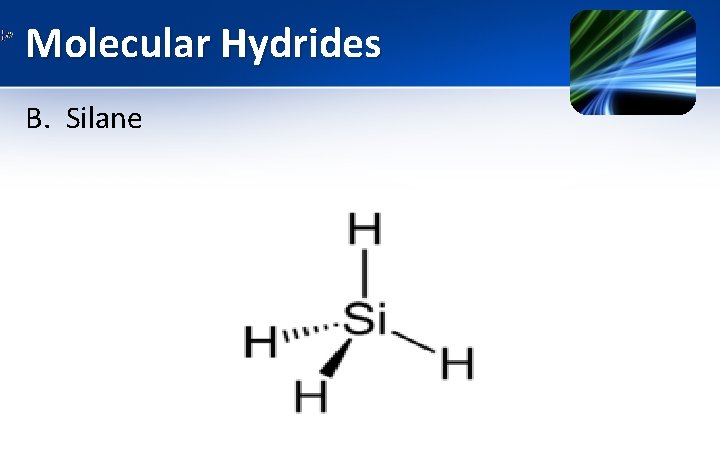
Molecular Hydrides B. Silane (Si. H 4) • Si: At No. 14: 1 S 2 2 P 6 3 S 2 3 Px 1 3 Py 1 3 Pz 0 • Group 14 element • Silane has tetrahedral structure (tetravalent) • SP 3 hybridized

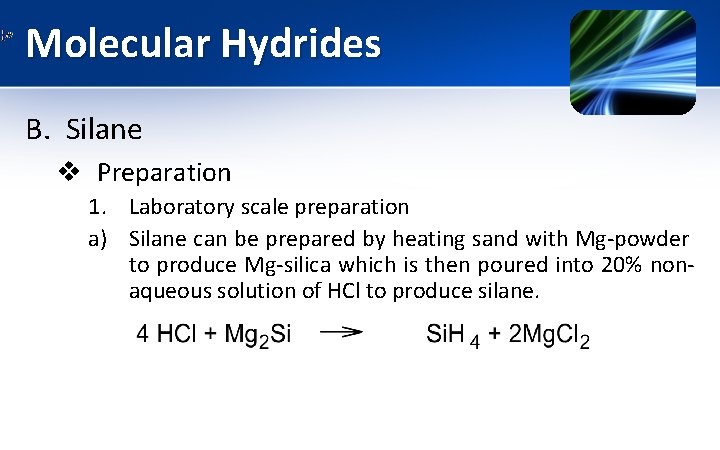
Molecular Hydrides B. Silane v Preparation 1. Laboratory scale preparation a) Silane can be prepared by heating sand with Mg-powder to produce Mg-silica which is then poured into 20% nonaqueous solution of HCl to produce silane.

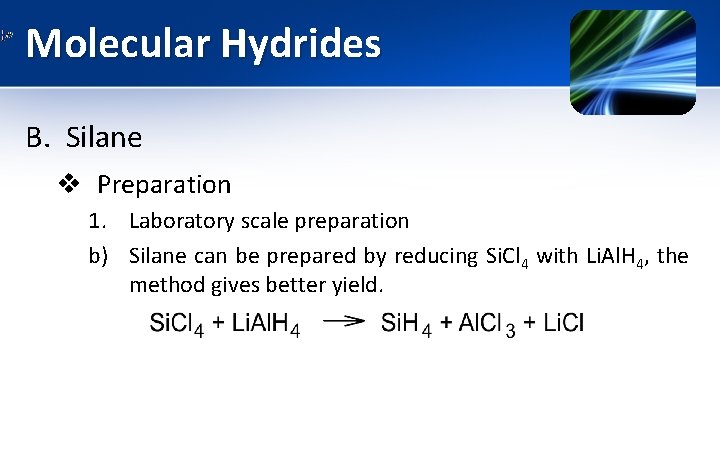
Molecular Hydrides B. Silane v Preparation 1. Laboratory scale preparation b) Silane can be prepared by reducing Si. Cl 4 with Li. Al. H 4, the method gives better yield.

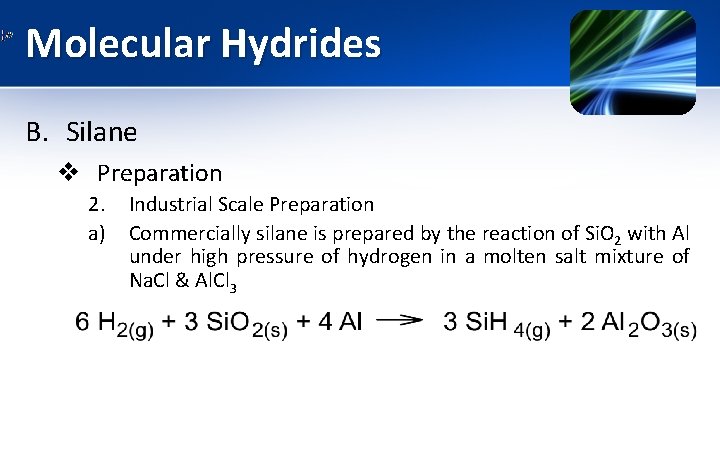
Molecular Hydrides B. Silane v Preparation 2. a) Industrial Scale Preparation Commercially silane is prepared by the reaction of Si. O 2 with Al under high pressure of hydrogen in a molten salt mixture of Na. Cl & Al. Cl 3

Molecular Hydrides B. Silane v Preparation 2. Industrial Scale Preparation c) It can also be prepared by the reaction of Li. H with silicon tetrachloride.

Molecular Hydrides B. Silane v Preparation 2. Industrial Scale Preparation d) Silane can be produced from metallurgical grade silicon in two step process. In 1 st step, powdered Si is reacted with HCl at 300 o. C to produce trichlorosilane along with H 2 gas. In 2 nd step, trichlorosilane is then boiled on resinous bed. .

Molecular Hydrides B. Silane (Properties) 1. 2. 3. 4. 5. Silane has tetrahedral structure. It is a colorless gas. Silanes are much more reactive than alkanes. Si. H 4 is spontaneously flammable in air. Strong reducing agent. They react explosively with halogens at 250 C 6. Silane b. p. = 1110 C

Molecular Hydrides B. Silane Applications 1. Silane is used in the production of semiconductor devices such as solar cells. Silanes are used in applying polycrystalline silicon layers on silicon wafers while manufacturing semiconductors. 2. Low cost solar panels can be prepared by using silane which is used for depositing amorphous silicon on glass or other tubes. 3. It can be used as water repellants.

Molecular Hydrides B. Silane Applications 4. Silanes are used in masonry protection. 5. It is used as sealants. 6. It is used as coupling agents to adhere glass fibres to a polymer matrix stabilizing the composite material. 7. Used as strong reducing agent. 8. In preparation of halogensilane.

Molecular Hydrides C. Germane

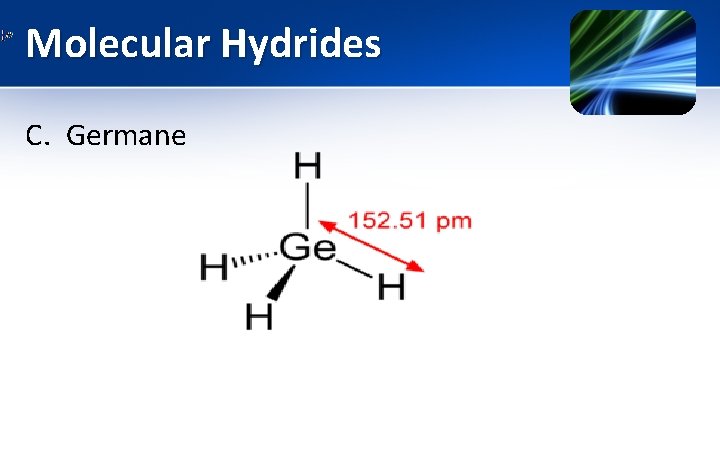
Molecular Hydrides C. Germane (Ge. H 4) • Group 14 element • Germane is tetravalent

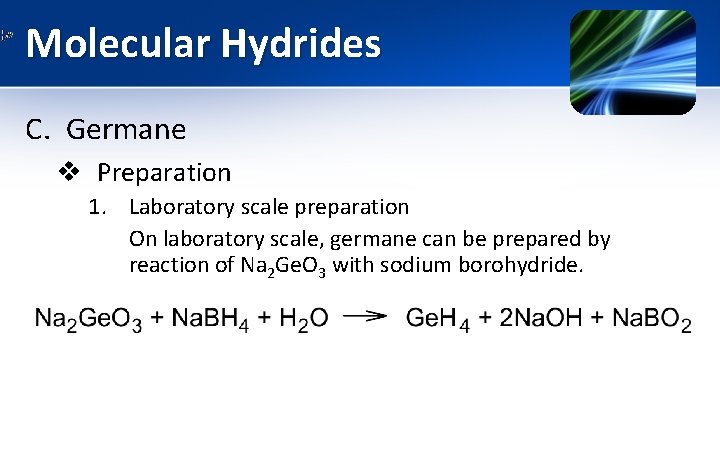
Molecular Hydrides C. Germane v Preparation 1. Laboratory scale preparation On laboratory scale, germane can be prepared by reaction of Na 2 Ge. O 3 with sodium borohydride.

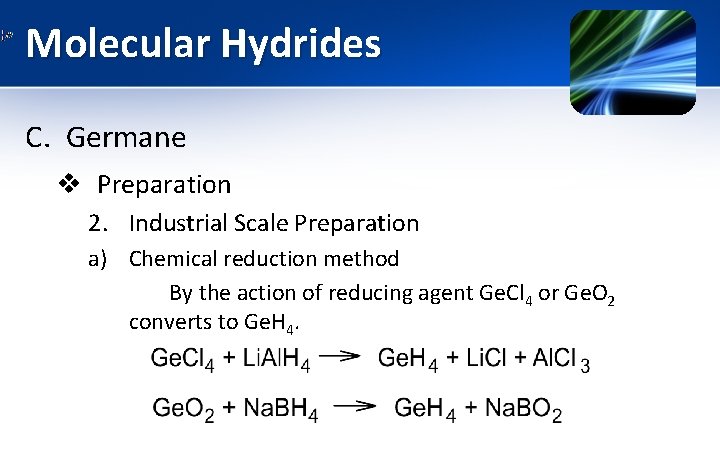
Molecular Hydrides C. Germane v Preparation 2. Industrial Scale Preparation a) Chemical reduction method By the action of reducing agent Ge. Cl 4 or Ge. O 2 converts to Ge. H 4.

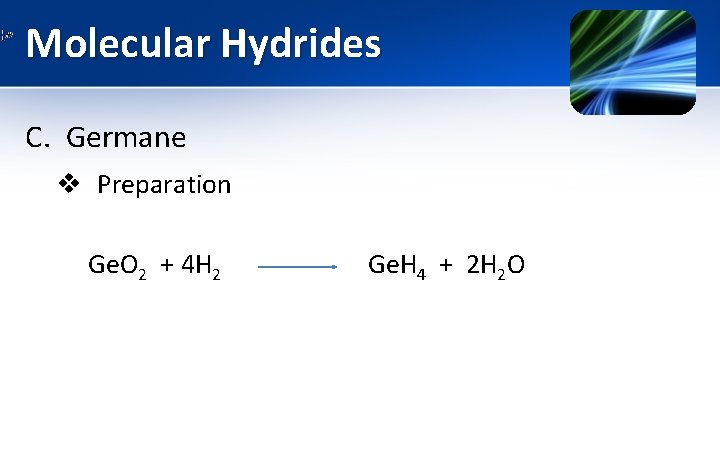
Molecular Hydrides C. Germane v Preparation Ge. O 2 + 4 H 2 Ge. H 4 + 2 H 2 O

Molecular Hydrides C. Germane (Properties) 1. It is the simplest germanium hydride & is the most useful compound of Germanium. 2. It is having similar tetrahedral structure as that of methane & silane. 3. It is a colourless gas. 4. It burns in air to give Ge. O 2 & water.

Molecular Hydrides C. Germane (Properties) 5. They are similar to silane, but are less volatile, less flammable & are unaffected by water or aqueous acid or alkali. (Resistant to hydrolysis even by 30% Na. OH) 6. Germane: b. p. = 880 C

Molecular Hydrides C. Germane (Applications) Germane finds applications in semiconductor industry.

Saline / Ionic Hydrides v These are formed by metals which are more electropositive than hydrogen. Such metals are of group I & group II except Be & Mg. v These are formed by transfer of electrons from metal to hydrogen atom & contains H ions. v e. g. Li. H, Na. H, Ca. H 2, etc

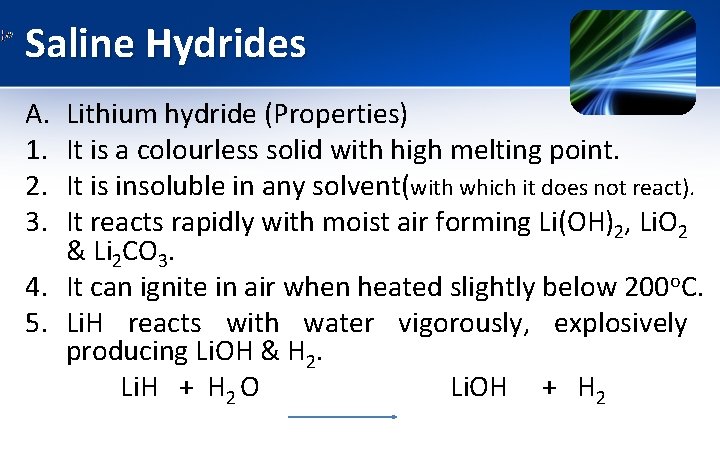
Saline/ Ionic Hydrides A. Lithium hydride (Li. H) It is prepared by direct heating of lithium metal with hydrogen gas at temperature above 600 o. C. Increase in temperature or pressure, addition of carbon upto 0. 003%, increases the yield upto 98%.

Saline Hydrides A. 1. 2. 3. Lithium hydride (Properties) It is a colourless solid with high melting point. It is insoluble in any solvent(with which it does not react). It reacts rapidly with moist air forming Li(OH)2, Li. O 2 & Li 2 CO 3. 4. It can ignite in air when heated slightly below 200 o. C. 5. Li. H reacts with water vigorously, explosively producing Li. OH & H 2. Li. H + H 2 O Li. OH + H 2

Saline Hydrides A. Lithium hydride Applications 1. It is used for storage of hydrogen (12. 5%)but this application is restricted because of high stability. Removal of H 2 requires high temperature above 700 o. C. 2. It acts as reducing agent -it can be used in the production of variety of reagents like Li. Al. H 4, Na. BH 4. 3. Li. H is used for shielding in nuclear reactors.

Saline / Ionic Hydrides B. Sodium hydride (Na. H) It is prepared by direct heating of sodium metal with hydrogen gas at high temperature close to 700 o. C.

Saline / Ionic Hydrides B. 1. 2. 3. 4. Sodium hydride (Properties) It is a colorless solid with high melting point. It is insoluble in organic solvent. It can ignite in air. It gives explosively violent reaction with water.

Saline Hydrides B. Sodium hydride Applications 1. It acts as a strong reducing agent reducing various compounds. 2. It can be used to dry some organic solvents because of its quick & irreversible reaction with water. 3. Na. H pellets when crushed in the presence of water releases H 2. Therefore Na. H is proposed for H 2 storage for the use in fuel cell vehicles. 4. It is used for preparing Na. BH 4.

Storage of Hydrogen A. Chemical Storage: In the form of metal hydride as Sodium Alanates B. Physical Storage: via compression, liquification, adsorption on porous carbon materials.

A. Chemical Storage § Alanates are complex metal hydrides §These are metal aluminohydrides § Sodium alanate (Na. Al. H 4) is a promising material for H 2 storage with capacity of 5. 5 wt %. § It is one of the known complex used in PEM fuel cell which operates at about 80 o. C.

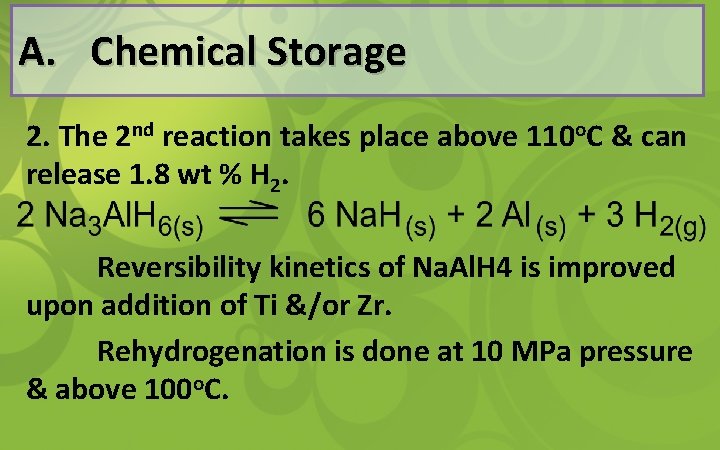
A. Chemical Storage H 2 is released from Na. Al. H 4 in the following steps: 1. At 1 atm pressure, the first reaction becomes thermodynamically favorable at temperature above 33 o. C & release 3. 7 wt % H 2. 3

A. Chemical Storage 2. The 2 nd reaction takes place above 110 o. C & can release 1. 8 wt % H 2. Reversibility kinetics of Na. Al. H 4 is improved upon addition of Ti &/or Zr. Rehydrogenation is done at 10 MPa pressure & above 100 o. C.

Limitations of Sodium Alanates 1. Storage of H 2 by alanates is slow, recovery of H 2 also slow. q It helps for fundamental understanding, designing & developing improved types of complex metal hydrides.

Other Metal Hydrides §Hydrides of Li, Na can also be prepared by interaction of hydrogen with the metal. §These metal hydrides release hydrogen by heating at suitable high temperature. §Li. H contain 12. 5% hydrogen. §Na. H contain 4. 1% hydrogen. §Ammonia-borane, metal borohydrides, etc.

B. Physical Storage §Carbon Material §Liquid Hydrogen §Cylinders

B. Physical Storage § Carbon Material: §Physical adsorption of H 2 on porous carbon material is one of the main methods being considered for automobile applications. § It can be done in several forms of carbon like amorphous activated carbon, graphite, nanotubes, etc.

B. Physical Storage § Carbon nanomaterials are in different forms: 1. Fullerene 2. Single walled carbon nanotubes 3. Multiwalled carbon nanotubes 4. Carbon & Graphite nanofibres

B. Physical Storage §Storage is done at high pressure & low temperature. §Hydrogen can be recovered by sucction or heating. §Amount of hydrogen stored depends upon carbon material, particle size, pressure, time, etc. § e. g. Based on surface area of single graphene sheet (1315 m 2/g), the storage capacity of hydrogen adsorbed on graphene is about 3. 3 % by weight at cryogenic temperature

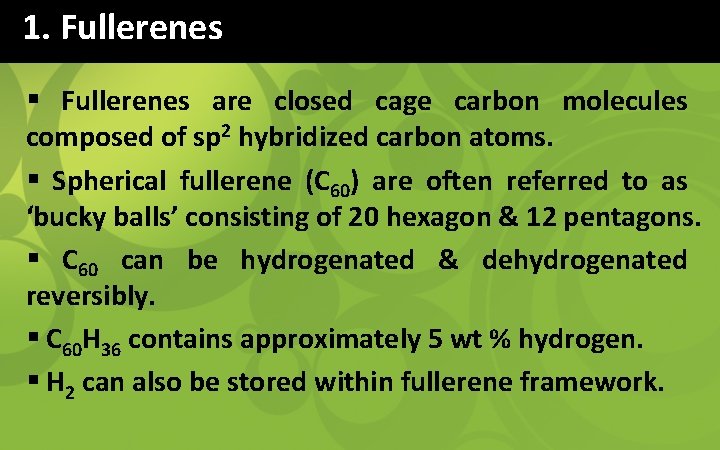
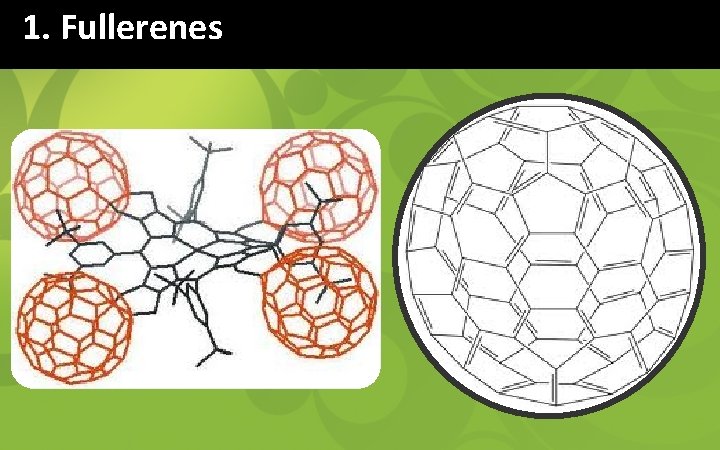
1. Fullerenes § Fullerenes are closed cage carbon molecules composed of sp 2 hybridized carbon atoms. § Spherical fullerene (C 60) are often referred to as ‘bucky balls’ consisting of 20 hexagon & 12 pentagons. § C 60 can be hydrogenated & dehydrogenated reversibly. § C 60 H 36 contains approximately 5 wt % hydrogen. § H 2 can also be stored within fullerene framework.

1. Fullerenes

2. Single walled carbon nanotubes (SWNT) § SWNT’s is like single rolled sheet of graphene. § It has a narrow porous size which makes them attractive as adsorbents of H 2. § H 2 uptake increases with surface area which increases with tube diameter. § Under 1 atm pressure, the amount of H 2 adsorbed in SWNT’s is small (< 1 wt %) where as, under cryogenic conditions from 1 – 2. 4 wt %.

2. Single walled carbon nanotubes (SWNT)

3. Multiwalled carbon nanotubes (SWNT) § MWNT’s consist of layers of concentric cylinders of graphene with hollow center. § The spacing between each cylinder is similar to the interplanar distance in graphite, with number of cells varying from 2 to 50. § MWNT’s are inactive for H 2 storage, but alkali doped MWNT’s can store H 2 of 7. 2 wt %.

3. Multiwalled carbon nanotubes (SWNT)

4. Carbon & Graphite Nanofibres § Carbon nanofibres (CNF’s) are layered graphitic nanostructures. §Graphite nanofibres (GNF’s) consists of the stacks of graphene plates & cones, have plenty of open edges that can favor H 2 adsorption. §GNF produced by pyrolysis of acetylene adsorb 6. 5 wt % H 2.

4. Carbon Nanofibres

4. Graphite Nanofibres

B. Physical Storage §Liquid Hydrogen §At cryogenic temperature, stored as liquid hydrogen in specially designed flask called ‘Dewar Flask’.

B. Physical Storage §Cylinders §Stored as compressed hydrogen gas

Difficulties in Storage & Transportation of Hydrogen • Ignition temperature of hydrogen is lowest of all gases, it is highly inflammable- hence risky for storage and transportation. • Difficult to liquefy as its boiling point is very low ( -252. 6 o. C)- high cost cooling required for the liquid hydrogen. technology is

Difficulties in Storage & Transportation of Hydrogen • Hydrogen storage as metal hydrides requires long time and high temperature. Recovery of hydrogen also requires high temperature. • It is lightest gas because of low Mol. Wt. (2)hence under pressure only small quantity of gas can be stored in cylinders (22. 4 lit of any gas at STP = Mol. Wt. )

Applications of Hydrogen (a) Fuel Cells : hydrogen is fuel in fuel cell. Also to generate electricity. (b) Industrial Use : (i) For welding and brazing (at high temperature). (ii) For annealing of metals. (iii) For cutting of metals under water. (iv) For fabrication of quartz and glass (oxy-hydrogen flames are used). (v) for softening of food industries.

Applications of Hydrogen (c) Aviation Fuel : Liquid hydrogen is used in missiles and rockets (as rocket propellant) due to its high energy content per unit mass and light weight. (d) Reducing agent (e)Manufacture of ammonia industries), CH 3 OH, HCHO, etc. (Used in fertilizer
 Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
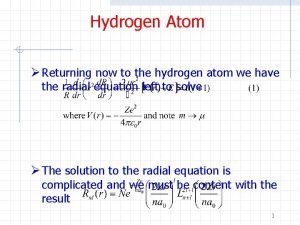
Inorganic vs organic chemistry Hydrogen schrodinger equation
Hydrogen schrodinger equation Hydrogen isotopes
Hydrogen isotopes Larmor frequency formula
Larmor frequency formula Star
Star Lunar polar hydrogen mapper
Lunar polar hydrogen mapper H - c
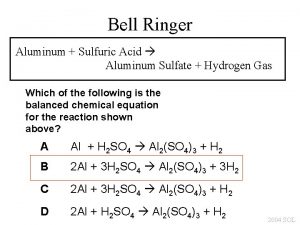
H - c H2so4+al
H2so4+al Fuel cell forklift
Fuel cell forklift Atomic emmision spectrum
Atomic emmision spectrum A saturated fatty acid holds all the hydrogen atoms it can.
A saturated fatty acid holds all the hydrogen atoms it can. Symmetrical alkene
Symmetrical alkene Bohr radius of hydrogen atom
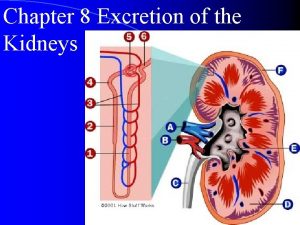
Bohr radius of hydrogen atom Hydrogen secretion in kidney
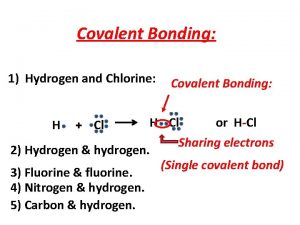
Hydrogen secretion in kidney Covalent bond hydrogen and chlorine
Covalent bond hydrogen and chlorine Aluminum bohr diagram
Aluminum bohr diagram Word equation of combustion of hydrogen
Word equation of combustion of hydrogen In bohr theory of hydrogen atom let r v
In bohr theory of hydrogen atom let r v Number of protons in hydrogen
Number of protons in hydrogen Hydrogen strategy
Hydrogen strategy Mri hydrogen atoms
Mri hydrogen atoms Hydrogen spectrum
Hydrogen spectrum Percent composition
Percent composition Hydrogen strategies
Hydrogen strategies Atom palm hydrogen
Atom palm hydrogen Chlorine and hydrogen covalent bond
Chlorine and hydrogen covalent bond Hydrogen storage
Hydrogen storage Hydrogen bond
Hydrogen bond Hydrogen isotope
Hydrogen isotope Dot and cross diagram of carbon dioxide
Dot and cross diagram of carbon dioxide Molecules containing hydrogen
Molecules containing hydrogen Standard electrode potential
Standard electrode potential Hydrochloric acid and sodium bicarbonate
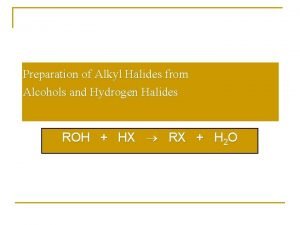
Hydrochloric acid and sodium bicarbonate Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Hydrogen-1
Hydrogen-1 Each column of the periodic table is
Each column of the periodic table is Onsite hydrogen generation
Onsite hydrogen generation Manganese oxide and hydrogen peroxide
Manganese oxide and hydrogen peroxide Hydrogen bromide intermolecular forces
Hydrogen bromide intermolecular forces Cecil writes the equation for the reaction of hydrogen
Cecil writes the equation for the reaction of hydrogen Atom palm hydrogen
Atom palm hydrogen Hydrogen assisted cracking
Hydrogen assisted cracking Atomic mass of radon
Atomic mass of radon Monolith hydrogen
Monolith hydrogen Hydrogen spectrum
Hydrogen spectrum Physics words list
Physics words list Mri hydrogen atoms
Mri hydrogen atoms Hydrogen car development
Hydrogen car development Amines hydrogen bonding
Amines hydrogen bonding Addition of hydrogen halides
Addition of hydrogen halides What is effective nuclear charge
What is effective nuclear charge Addition of hydrogen halides to alkynes
Addition of hydrogen halides to alkynes Nelson curve api 941
Nelson curve api 941 Hydrogen mass number
Hydrogen mass number Hydrogen bomb vs atomic bomb
Hydrogen bomb vs atomic bomb Alkaline fuel cell animation
Alkaline fuel cell animation Hydrogen valley denmark
Hydrogen valley denmark Binary hydrogen compounds
Binary hydrogen compounds Secondary structure of protein bond
Secondary structure of protein bond Argon hyphen notation
Argon hyphen notation Classification of hydrogen
Classification of hydrogen Xkcd exponential
Xkcd exponential Zinc dissolves in hydrochloric acid to yield hydrogen gas
Zinc dissolves in hydrochloric acid to yield hydrogen gas Energy storage association
Energy storage association Production of hydrogen from methane
Production of hydrogen from methane Amines hydrogen bonding
Amines hydrogen bonding Physical properties of hydrogen sulphide
Physical properties of hydrogen sulphide Hydrogen plasma
Hydrogen plasma How to draw atoms
How to draw atoms Water is polar or nonpolar
Water is polar or nonpolar Corrosion oxidation or reduction
Corrosion oxidation or reduction Hydride vs hydrogen
Hydride vs hydrogen Balancing equations counting atoms
Balancing equations counting atoms Hard swell in canning
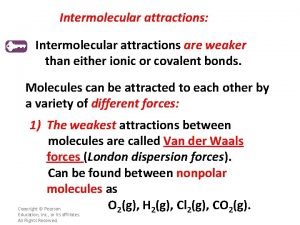
Hard swell in canning Hydrogen or covalent bond stronger
Hydrogen or covalent bond stronger Hydrogen gas
Hydrogen gas Unit 5 chemical reactions
Unit 5 chemical reactions Covalent bond vs hydrogen bond which is stronger
Covalent bond vs hydrogen bond which is stronger Hydrogen
Hydrogen Hydrogen motor
Hydrogen motor A human hair is approximately 50 μm in diameter
A human hair is approximately 50 μm in diameter 5 uses of hydrogen
5 uses of hydrogen H-bomb apush definition
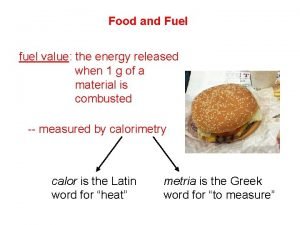
H-bomb apush definition Fuel value of food
Fuel value of food Metal salt + acid
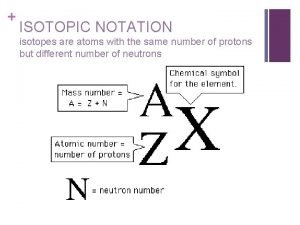
Metal salt + acid Lead isotopic notation
Lead isotopic notation Valence electrons oxygen
Valence electrons oxygen Hydrogen tank
Hydrogen tank Hcn lewis structure
Hcn lewis structure Addition of halogens to alkenes
Addition of halogens to alkenes Mengelas
Mengelas Skallmodell
Skallmodell Watercovalent bond
Watercovalent bond Alarp matrix
Alarp matrix