Chemical Reactions Mrs Wolfe I II IV V



- Slides: 14

Chemical Reactions Mrs. Wolfe I II IV V

Signs of a Chemical Reaction Change in heat and light · Exothermic – GIVES OFF heat · Endothermic – ABSORBS heat n Formation of a gas n Formation of a precipitate n Color change n

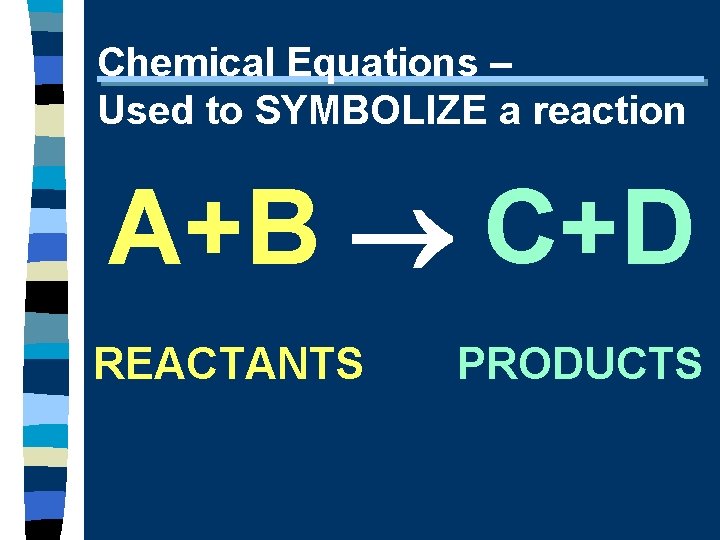
Chemical Equations – Used to SYMBOLIZE a reaction A+B C+D REACTANTS PRODUCTS

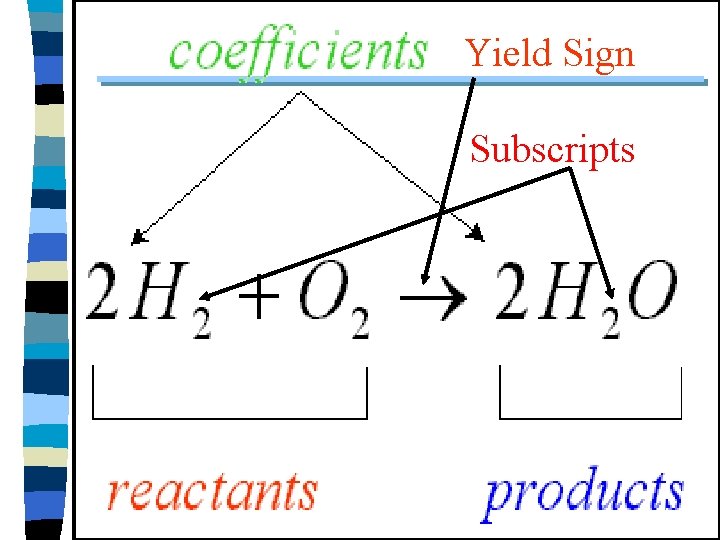
Chemical Equation Yield Sign Subscripts

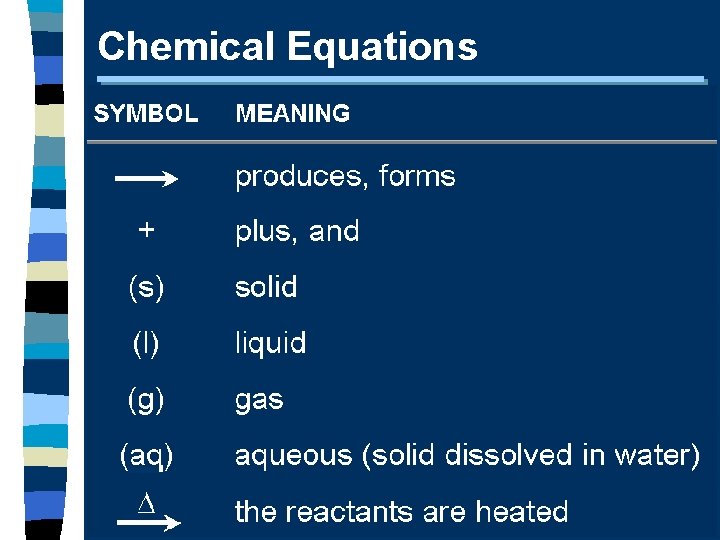
Chemical Equations

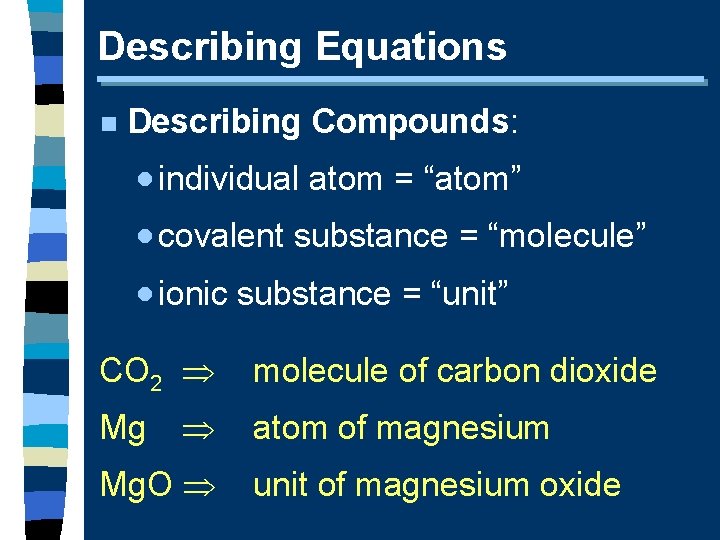
Describing Equations n Describing Compounds: · individual atom = “atom” · covalent substance = “molecule” · ionic substance = “unit” CO 2 Mg Mg. O molecule of carbon dioxide atom of magnesium unit of magnesium oxide

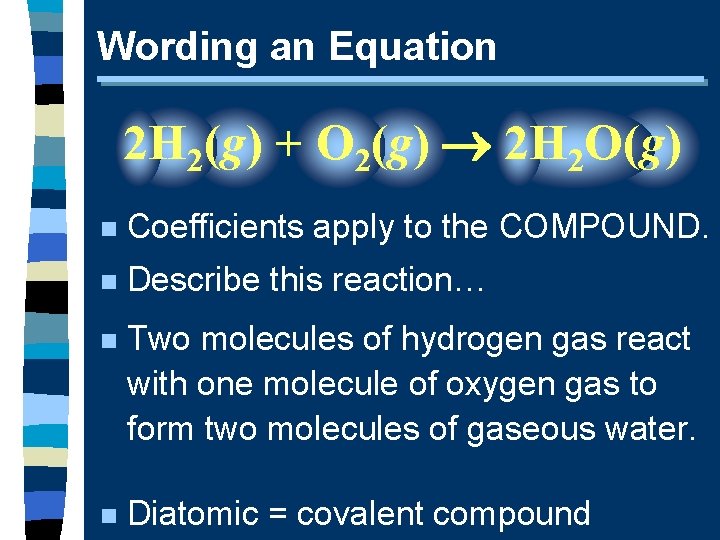
Wording an Equation 2 H 2(g) + O 2(g) 2 H 2 O(g) n Coefficients apply to the COMPOUND. n Describe this reaction… n Two molecules of hydrogen gas react with one molecule of oxygen gas to form two molecules of gaseous water. n Diatomic = covalent compound

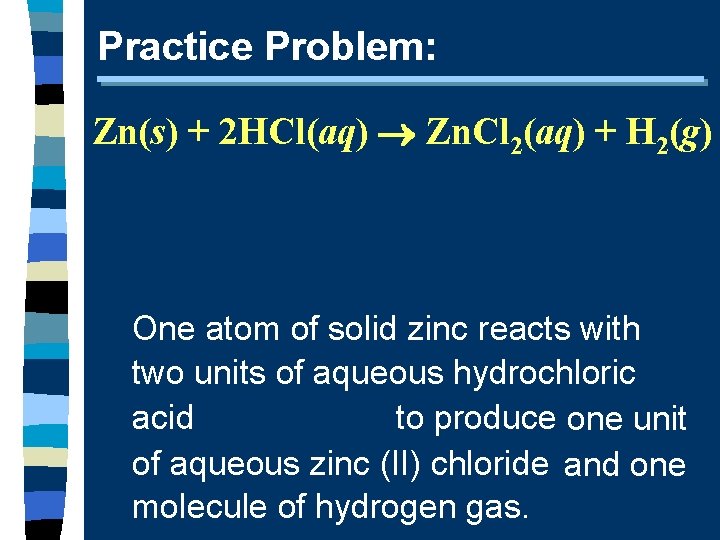
Practice Problem: Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) One atom of solid zinc reacts with two units of aqueous hydrochloric to produce one unit acid of aqueous zinc (II) chloride and one molecule of hydrogen gas.

Writing Equations Two atoms of aluminum react with three units of aqueous copper(II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride. 2 Al(s) + 3 Cu. Cl 2(aq) 3 Cu(s) + 2 Al. Cl 3(aq)

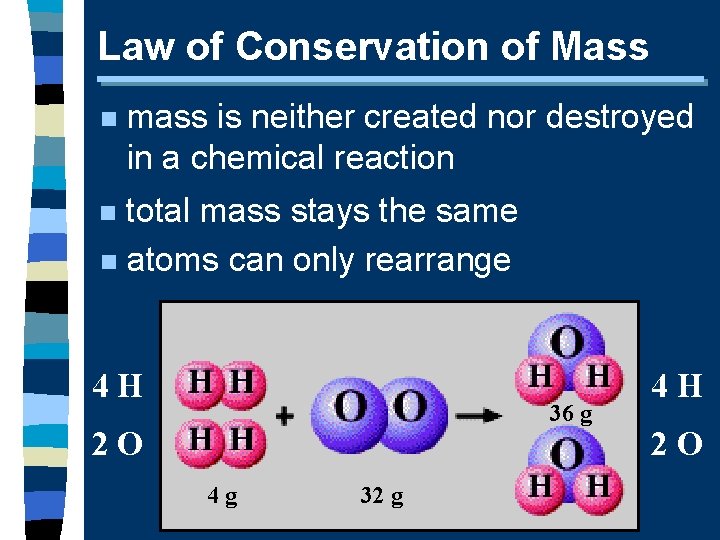
Law of Conservation of Mass n mass is neither created nor destroyed in a chemical reaction total mass stays the same n atoms can only rearrange n 4 H 36 g 2 O 4 g 32 g 4 H 2 O

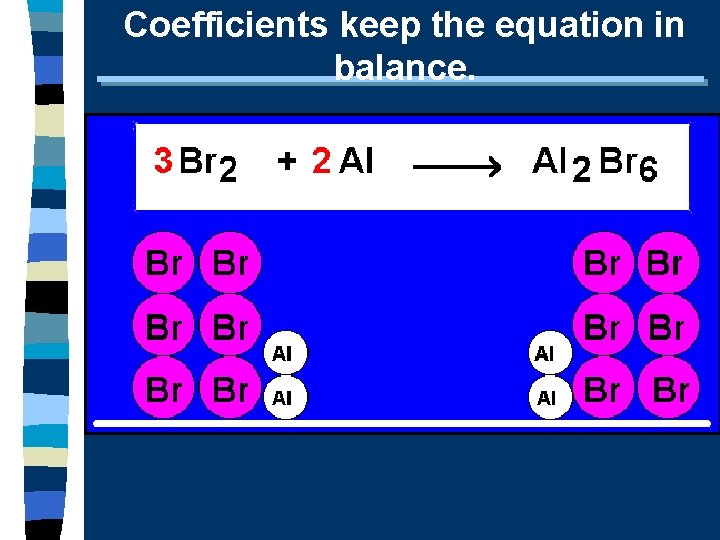
Coefficients keep the equation in balance.

A. Balancing Steps 1. Count ATOMS on each side. 3. Add coefficients to make #s equal. Coefficient subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!

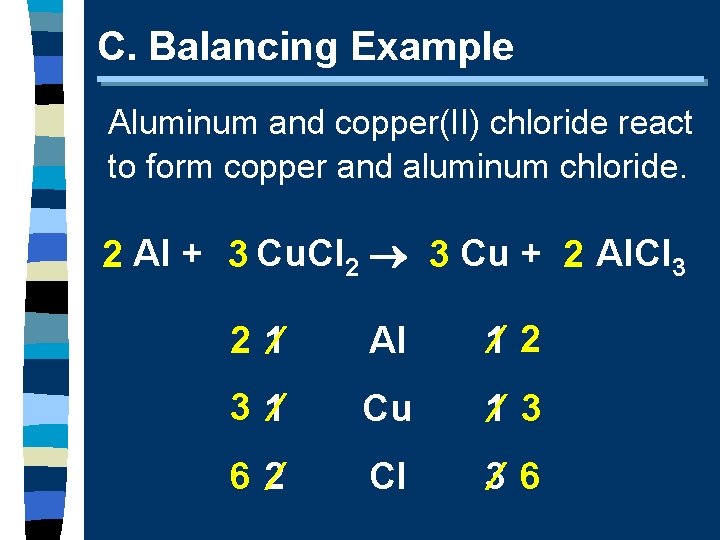
C. Balancing Example Aluminum and copper(II) chloride react to form copper and aluminum chloride. 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6

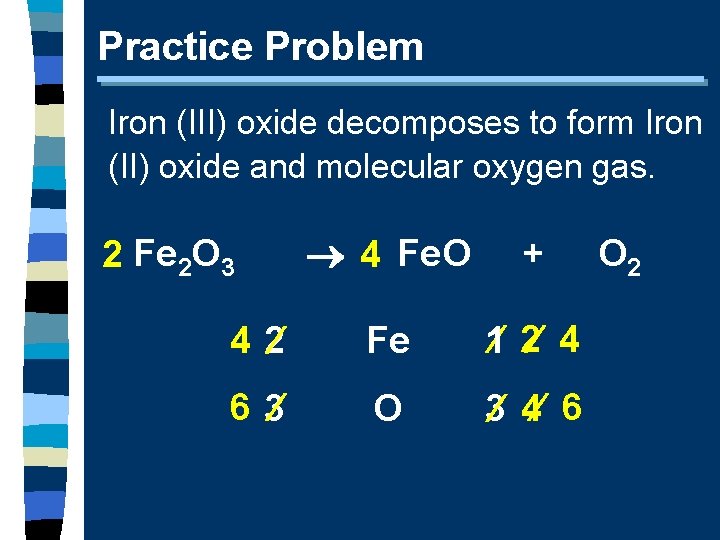
Practice Problem Iron (III) oxide decomposes to form Iron (II) oxide and molecular oxygen gas. 2 4 Fe. O + 4 2 Fe 1 2 4 6 3 O 3 4 6 2 Fe 2 O 3 O 2
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Redox reactions examples
Redox reactions examples Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet They are mrs garcia and mrs castro
They are mrs garcia and mrs castro They are mrs garcia and mrs castro
They are mrs garcia and mrs castro Mrs. darling was ___________ of mrs. s.
Mrs. darling was ___________ of mrs. s. Describing chemical reactions worksheet answers
Describing chemical reactions worksheet answers Are enzymes tertiary or quaternary
Are enzymes tertiary or quaternary Predict the products of the following reactions.
Predict the products of the following reactions. Solvent in chemical reactions
Solvent in chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions



























