CHEMICAL REACTIONS LIVING CELLS DEPEND ON THEM chemical

- Slides: 15

CHEMICAL REACTIONS LIVING CELLS DEPEND ON THEM!

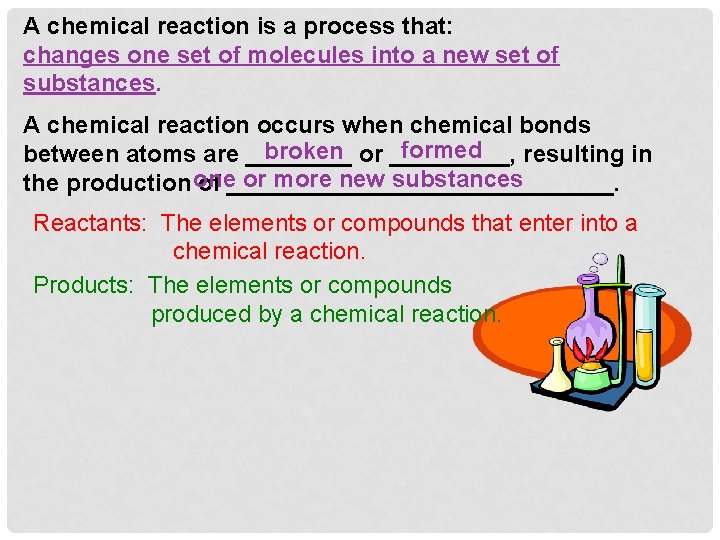
chemical reactions Life depends upon the __________ that occur within the cell. Chemical Reactions …. Amazing topic! Let’s get started!! These reactions are important to the: growth, development and the very survival of a cell. building of molecules, The reactions of a cell involve both the _______ breaking down and the ___________ of molecules. SPEED of these The role of enzymes is to greatly enhance the ______ reactions.

A chemical reaction is a process that: changes one set of molecules into a new set of substances. A chemical reaction occurs when chemical bonds formed broken or _____, between atoms are ____ resulting in or more new substances the production one of _______________. Reactants: The elements or compounds that enter into a chemical reaction. Products: The elements or compounds produced by a chemical reaction.

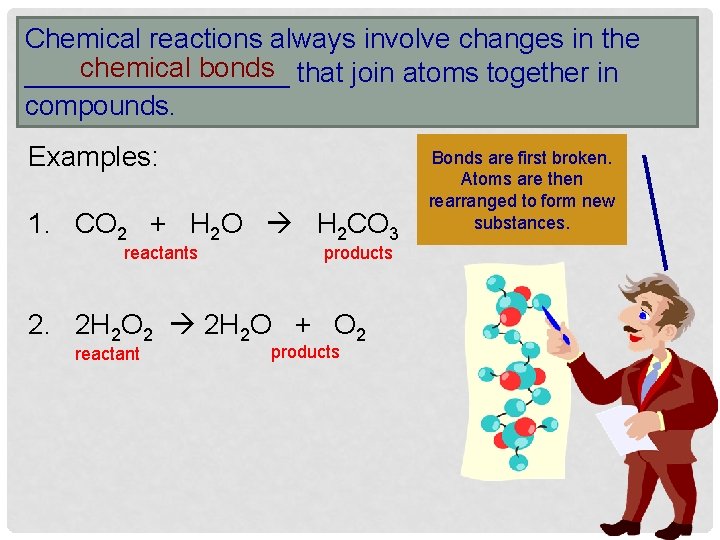
Chemical reactions always involve changes in the chemical bonds that join atoms together in _________ compounds. Examples: 1. CO 2 + H 2 O H 2 CO 3 reactants products 2. 2 H 2 O 2 2 H 2 O + O 2 reactant products Bonds are first broken. Atoms are then rearranged to form new substances.

Whenever chemical bonds form or are broken, energy will be released or absorbed ___________ __. The forming and breaking of bonds involves changes in energy. Energy in Reactions Some chemical reactions ____ absorb energy. Other chemical reactions _____ energy. release

Living organisms carry out a great variety of chemical reactions. Many of these reactions release energy, while many others absorb energy. Regardless of whether energy is released or absorbed by the reaction, starting the chemical reaction: requires an initial investment in energy called activation energy. In order for the reaction’s _____ products to form, existing _________ chemical bonds in the reactants must first be ____. broken This will require ____. energy

Activation Energy 1. The energy needed by the reactants in order to start a reaction. 2. It is the initial investment of energy for starting a reaction. 3. It is the energy required to break bonds in the reactant molecules.

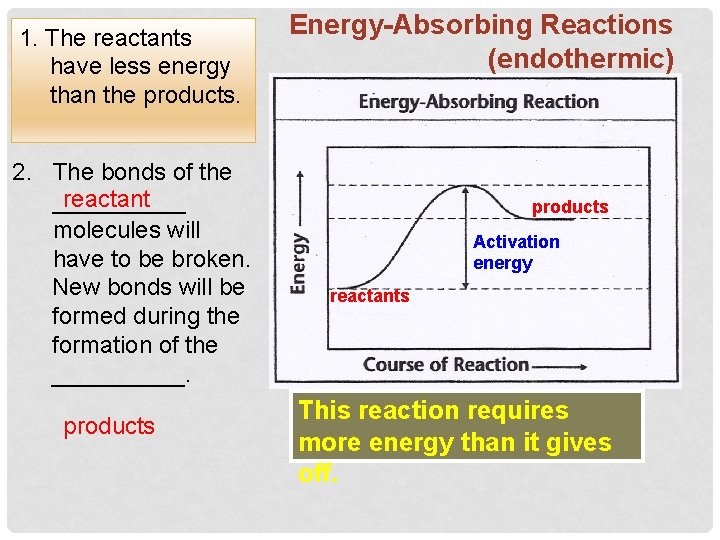
1. The reactants have less energy than the products. 2. The bonds of the reactant _____ molecules will have to be broken. New bonds will be formed during the formation of the _____. products Energy-Absorbing Reactions (endothermic) products Activation energy reactants This reaction requires more energy than it gives off.

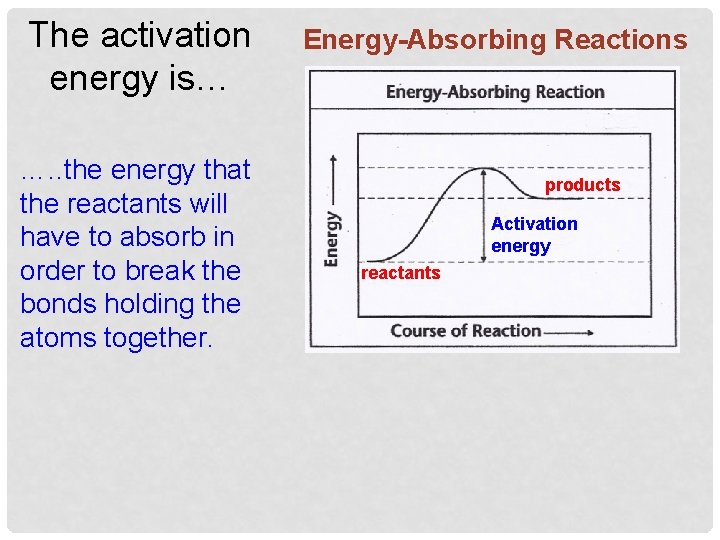
The activation energy is… …. . the energy that the reactants will have to absorb in order to break the bonds holding the atoms together. Energy-Absorbing Reactions products Activation energy reactants

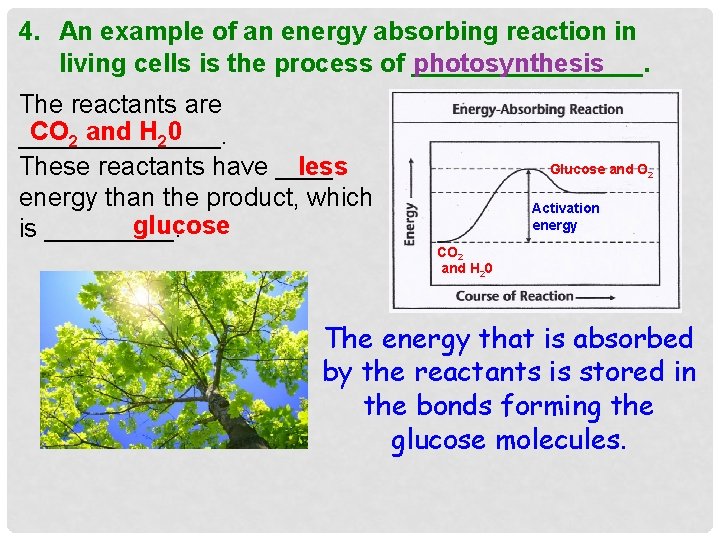
4. An example of an energy absorbing reaction in living cells is the process of ________. photosynthesis The reactants are CO 2 and H 20 _______. These reactants have ____ less energy than the product, which glucose is _____. Glucose and O 2 Activation energy CO 2 and H 20 The energy that is absorbed by the reactants is stored in the bonds forming the glucose molecules.

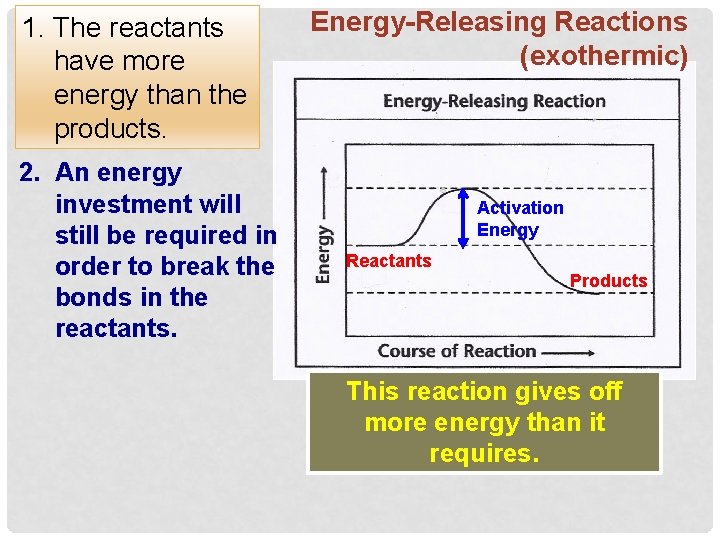
1. The reactants have more energy than the products. 2. An energy investment will still be required in order to break the bonds in the reactants. Energy-Releasing Reactions (exothermic) Activation Energy Reactants Products This reaction gives off more energy than it requires.

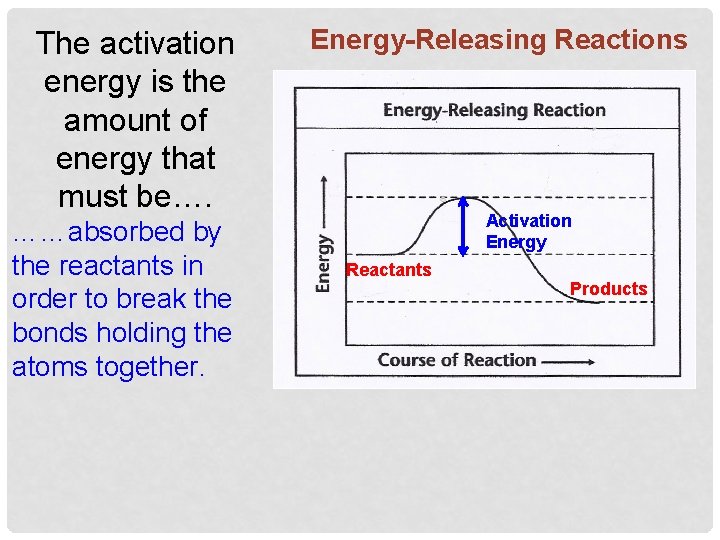
The activation energy is the amount of energy that must be…. ……absorbed by the reactants in order to break the bonds holding the atoms together. Energy-Releasing Reactions Activation Energy Reactants Products

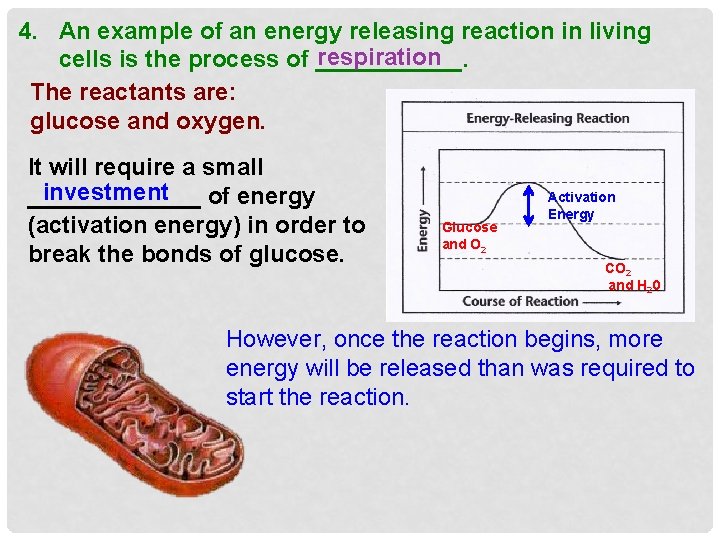
4. An example of an energy releasing reaction in living respiration cells is the process of ______. The reactants are: glucose and oxygen. It will require a small investment _______ of energy (activation energy) in order to break the bonds of glucose. Glucose and O 2 Activation Energy CO 2 and H 20 However, once the reaction begins, more energy will be released than was required to start the reaction.

This activation energy is usually in heat the form of _____ that the reactant molecules absorb from the surroundings _______. The bonds of the reactants break only when the molecules have: absorbed enough energy to become unstable. Activation energy is the amount of energy needed to push the reactants over an energy barrier or "hill" so that the "downhill" part of the reaction can begin.

Many of the chemical reactions of a cell proceed too _______slowly to be of use to the cell. The activation energy required for these reactions is simply too ______. high The cell must have a way to make these reactions occur ____faster and at lower temperatures ________. How is this done? ? ? ENZYMES!!
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Enzymes affect the reaction in living cells by changing the
Enzymes affect the reaction in living cells by changing the Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Physical properties of elements depend on
Physical properties of elements depend on Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal How to write redox half reactions
How to write redox half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet If you can t beat them join them
If you can t beat them join them Waters view with open mouth
Waters view with open mouth Medullary portion of collecting duct
Medullary portion of collecting duct Parafollicular cells vs follicular cells
Parafollicular cells vs follicular cells How are mitosis and meiosis similar
How are mitosis and meiosis similar Why dna is more stable than rna
Why dna is more stable than rna Chlorocruorin
Chlorocruorin