Chemical Reactions I Intro to Reactions 1 Turn


- Slides: 13

Chemical Reactions I. Intro to Reactions 1. Turn in Section 11. 1 to your class tray. 2. Get out paper for notes. I II IV V

Why are Chemical Reactions Important? Chemical reactions occur everywhere. n Your body is a chemical machine, if chemical reactions did not occur, we would not be alive. n

A. Signs of a Chemical Reaction Evolution of heat and light n Formation of a gas n Formation of a precipitate n Color change n

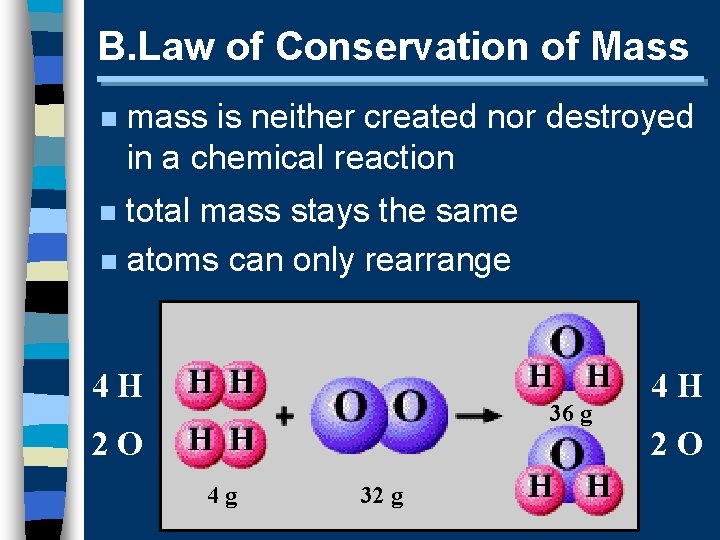
B. Law of Conservation of Mass n mass is neither created nor destroyed in a chemical reaction total mass stays the same n atoms can only rearrange n 4 H 36 g 2 O 4 g 32 g 4 H 2 O

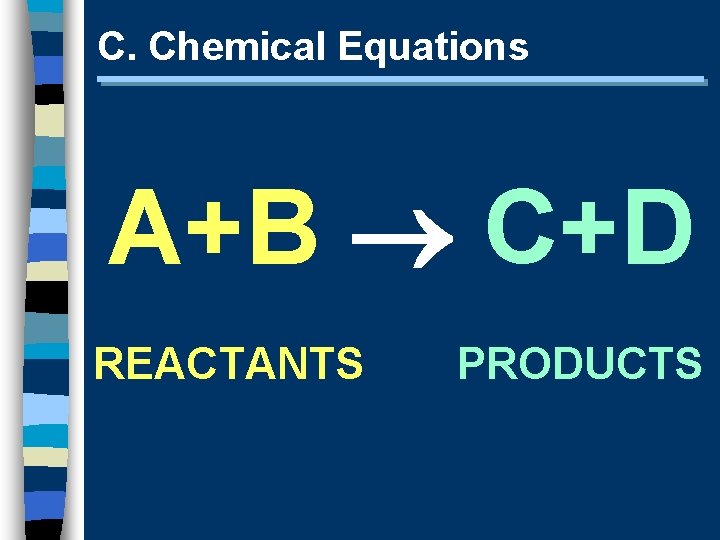
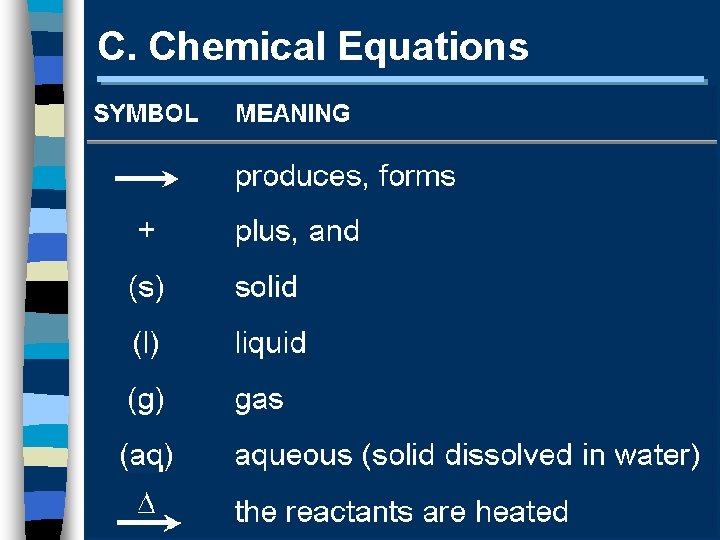
C. Chemical Equations A+B C+D REACTANTS PRODUCTS

C. Chemical Equations

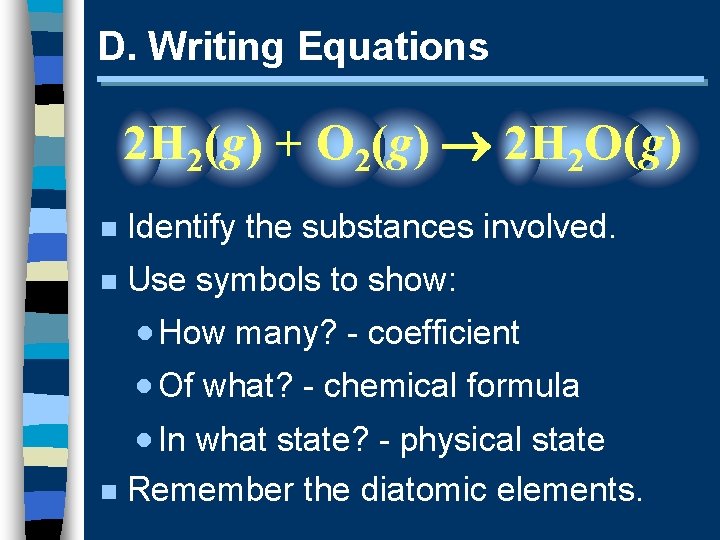
D. Writing Equations 2 H 2(g) + O 2(g) 2 H 2 O(g) n Identify the substances involved. n Use symbols to show: · How many? - coefficient · Of what? - chemical formula · In what state? - physical state n Remember the diatomic elements.

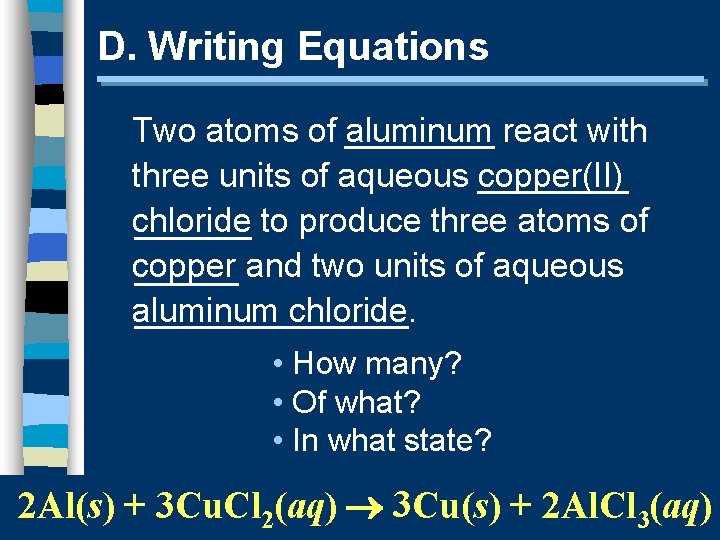
D. Writing Equations Two atoms of aluminum react with three units of aqueous copper(II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride. • How many? • Of what? • In what state? 2 Al(s) + 3 Cu. Cl 2(aq) 3 Cu(s) + 2 Al. Cl 3(aq)

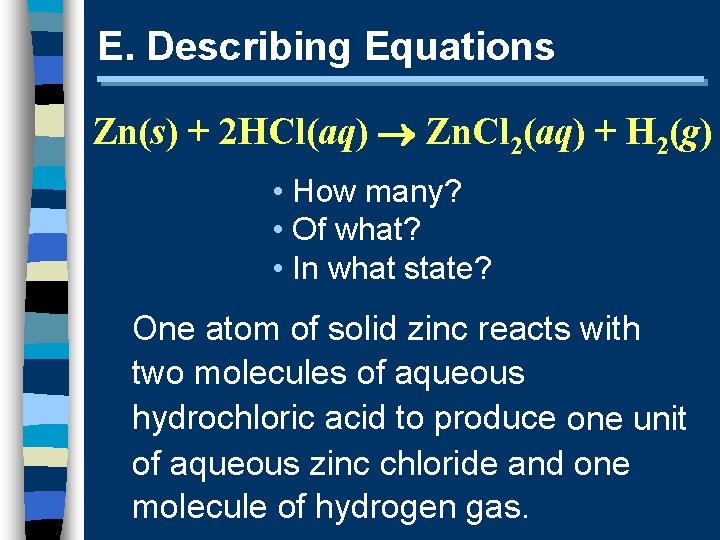
E. Describing Equations Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • How many? • Of what? • In what state? One atom of solid zinc reacts with two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas.

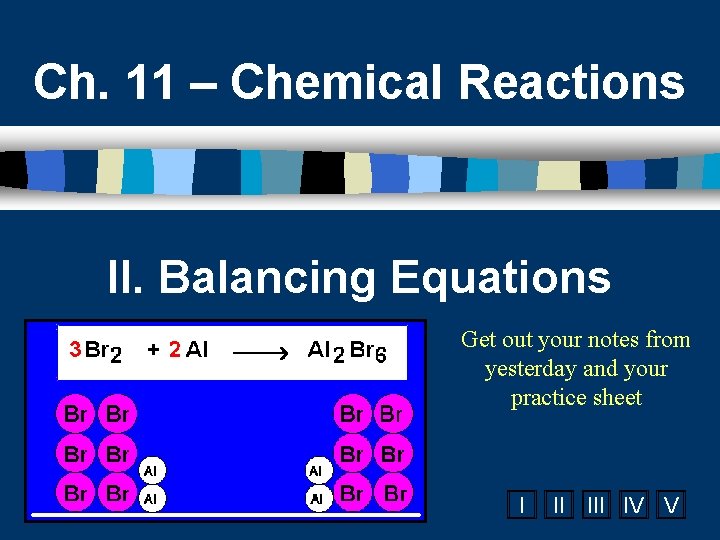
Ch. 11 – Chemical Reactions II. Balancing Equations Get out your notes from yesterday and your practice sheet I II IV V

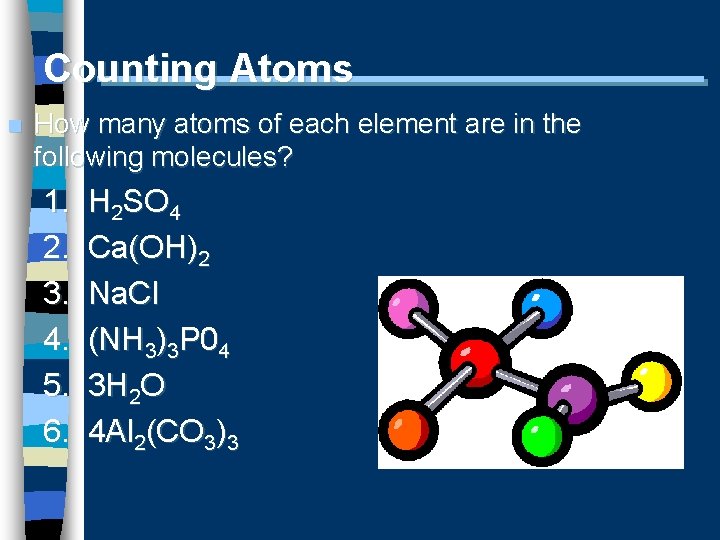
Counting Atoms n How many atoms of each element are in the following molecules? 1. 2. 3. 4. 5. 6. H 2 SO 4 Ca(OH)2 Na. Cl (NH 3)3 P 04 3 H 2 O 4 Al 2(CO 3)3

B. Helpful Tips Balance one element at a time. n Update ALL atom counts after adding a coefficient. n If an element appears more than once per side, balance it last. n Balance polyatomic ions as single units. · “ 1 SO 4” instead of “ 1 S” and “ 4 O” n

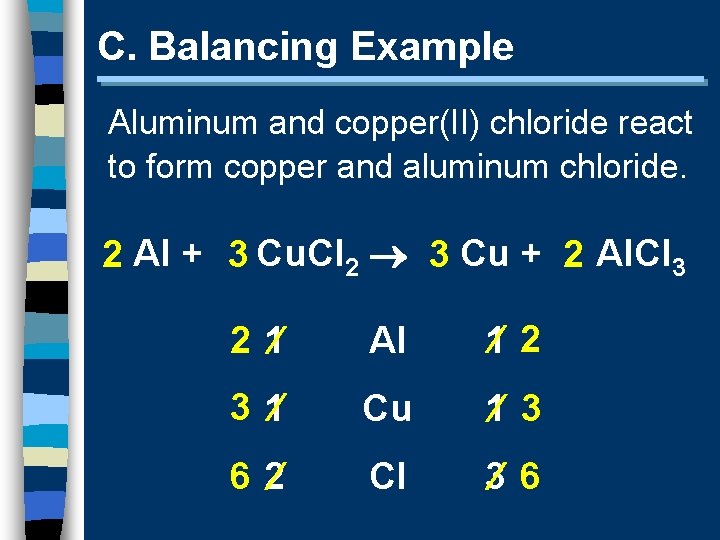
C. Balancing Example Aluminum and copper(II) chloride react to form copper and aluminum chloride. 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Are kc and kp equal
Are kc and kp equal Section 1 chemical changes
Section 1 chemical changes Go straight ahead and take the second left
Go straight ahead and take the second left Turn the arrow 1/4 turn clockwise
Turn the arrow 1/4 turn clockwise Themes about guilt
Themes about guilt You can't turn right here
You can't turn right here Worthy gentleman macbeth analysis
Worthy gentleman macbeth analysis A tomato flames
A tomato flames Turn hell hound turn
Turn hell hound turn Turn hell hound turn
Turn hell hound turn Walk straight and turn (1 نقطة) left right around
Walk straight and turn (1 نقطة) left right around

























