Chemical Calculations for Solutions Aqueous Solutions Much of




- Slides: 13

Chemical Calculations for Solutions

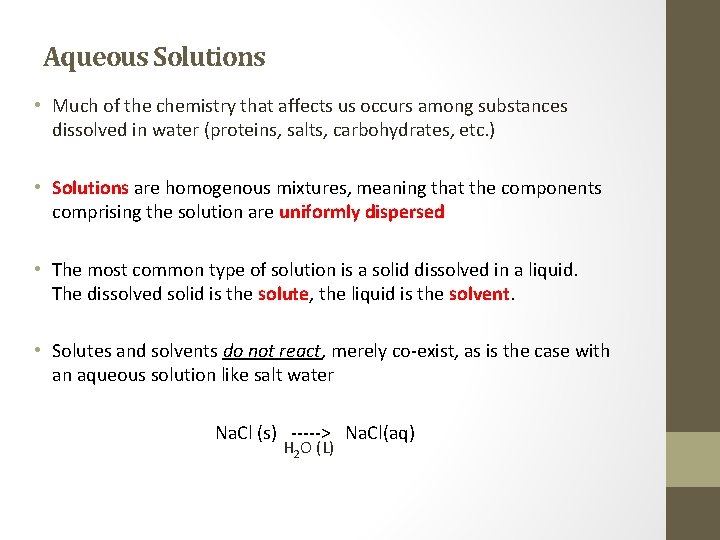
Aqueous Solutions • Much of the chemistry that affects us occurs among substances dissolved in water (proteins, salts, carbohydrates, etc. ) • Solutions are homogenous mixtures, meaning that the components comprising the solution are uniformly dispersed • The most common type of solution is a solid dissolved in a liquid. The dissolved solid is the solute, the liquid is the solvent. • Solutes and solvents do not react, merely co-exist, as is the case with an aqueous solution like salt water Na. Cl (s) -----> Na. Cl(aq) H 2 O (L)

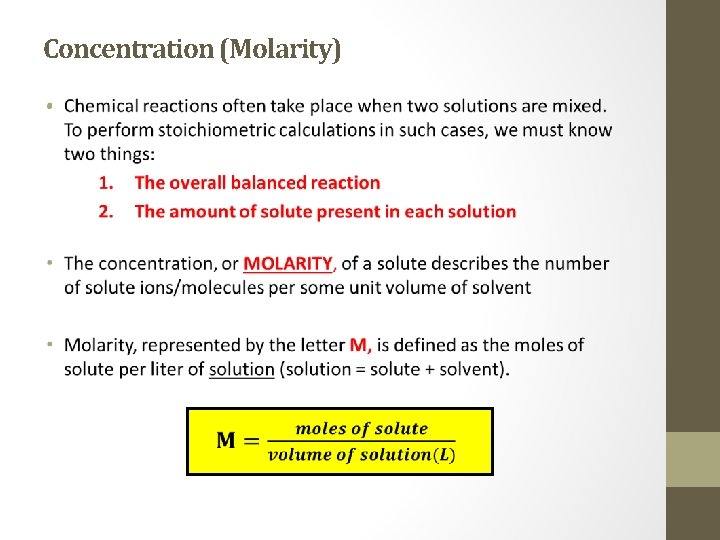
Concentration (Molarity) •


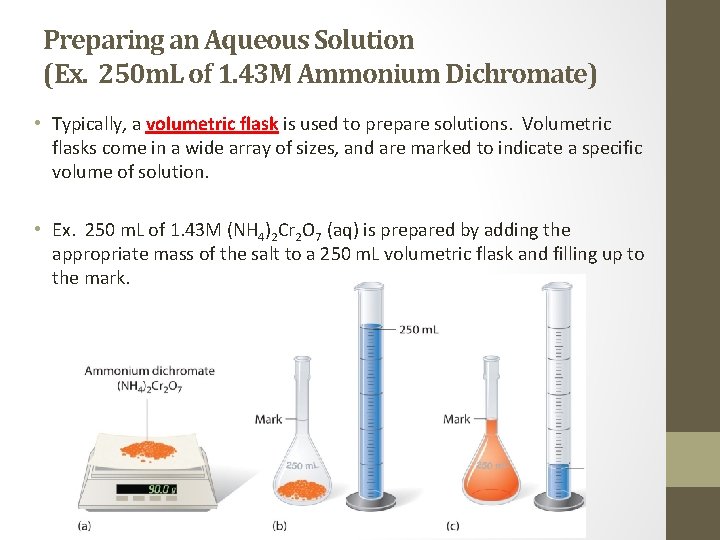
Preparing an Aqueous Solution (Ex. 250 m. L of 1. 43 M Ammonium Dichromate) • Typically, a volumetric flask is used to prepare solutions. Volumetric flasks come in a wide array of sizes, and are marked to indicate a specific volume of solution. • Ex. 250 m. L of 1. 43 M (NH 4)2 Cr 2 O 7 (aq) is prepared by adding the appropriate mass of the salt to a 250 m. L volumetric flask and filling up to the mark.

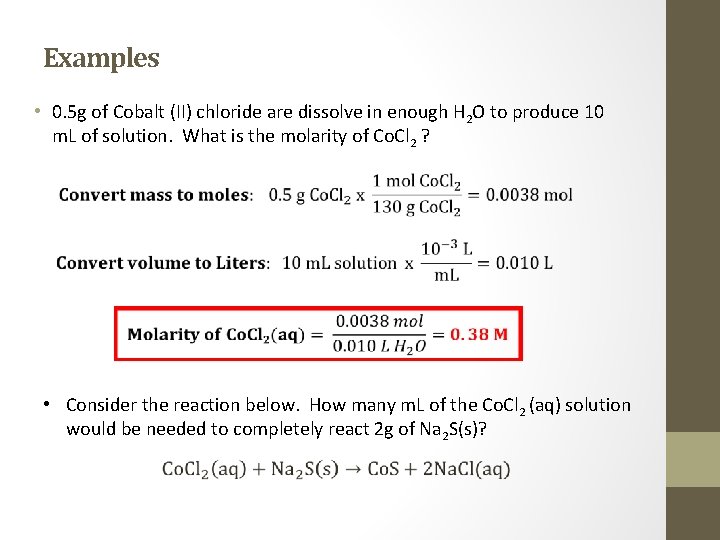
Examples • 0. 5 g of Cobalt (II) chloride are dissolve in enough H 2 O to produce 10 m. L of solution. What is the molarity of Co. Cl 2 ? • Consider the reaction below. How many m. L of the Co. Cl 2 (aq) solution would be needed to completely react 2 g of Na 2 S(s)?


Dilution • In many instances (especially in lab), you may need to prepare a solution of some desired concentration from a pre-existing stock solution. • For example, consider a concentrated detergent like Tide®. If wanted to wash a shirt, you wouldn’t just dump Tide® on it. • Instead, you add a cap-full or so (aliquot) to a large volume of water to attain a manageable solution. • This action of “watering down” the detergent to a useable state is called dilution. The bottle of Tide® is the stock solution.

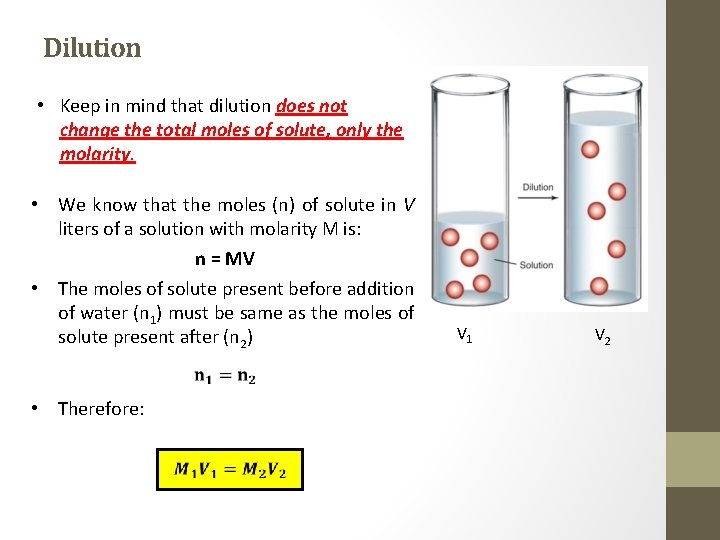
Dilution • Keep in mind that dilution does not change the total moles of solute, only the molarity. • We know that the moles (n) of solute in V liters of a solution with molarity M is: n = MV • The moles of solute present before addition of water (n 1) must be same as the moles of solute present after (n 2) • Therefore: V 1 V 2

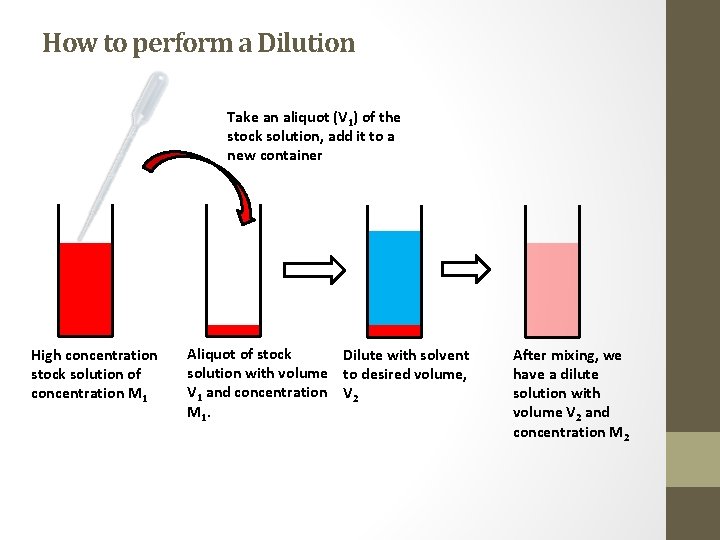
How to perform a Dilution Take an aliquot (V 1) of the stock solution, add it to a new container High concentration stock solution of concentration M 1 Aliquot of stock Dilute with solvent solution with volume to desired volume, V 1 and concentration V 2 M 1. After mixing, we have a dilute solution with volume V 2 and concentration M 2

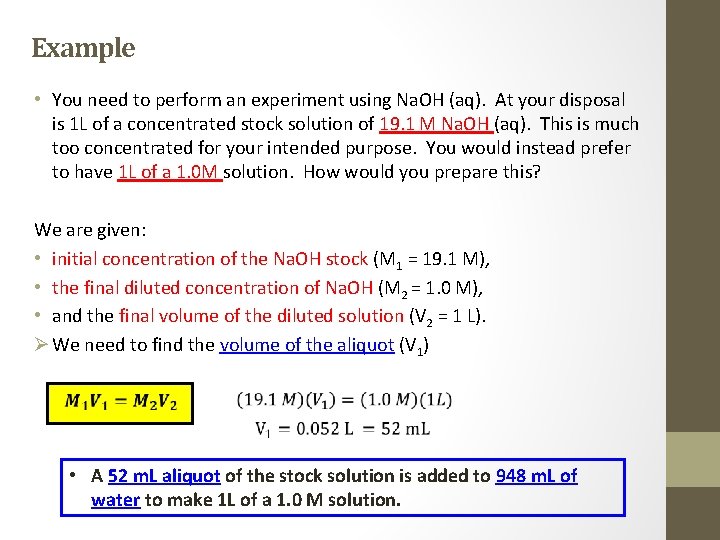
Example • You need to perform an experiment using Na. OH (aq). At your disposal is 1 L of a concentrated stock solution of 19. 1 M Na. OH (aq). This is much too concentrated for your intended purpose. You would instead prefer to have 1 L of a 1. 0 M solution. How would you prepare this? We are given: • initial concentration of the Na. OH stock (M 1 = 19. 1 M), • the final diluted concentration of Na. OH (M 2 = 1. 0 M), • and the final volume of the diluted solution (V 2 = 1 L). Ø We need to find the volume of the aliquot (V 1) • A 52 m. L aliquot of the stock solution is added to 948 m. L of water to make 1 L of a 1. 0 M solution.

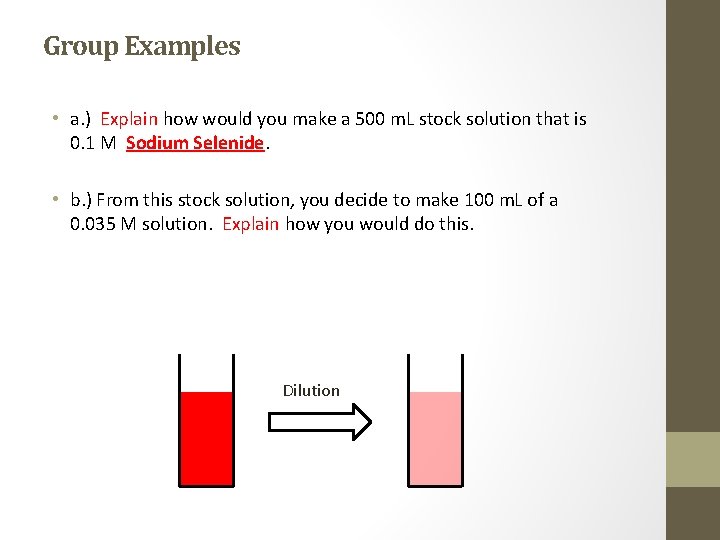
Group Examples • a. ) Explain how would you make a 500 m. L stock solution that is 0. 1 M Sodium Selenide. • b. ) From this stock solution, you decide to make 100 m. L of a 0. 035 M solution. Explain how you would do this. Dilution

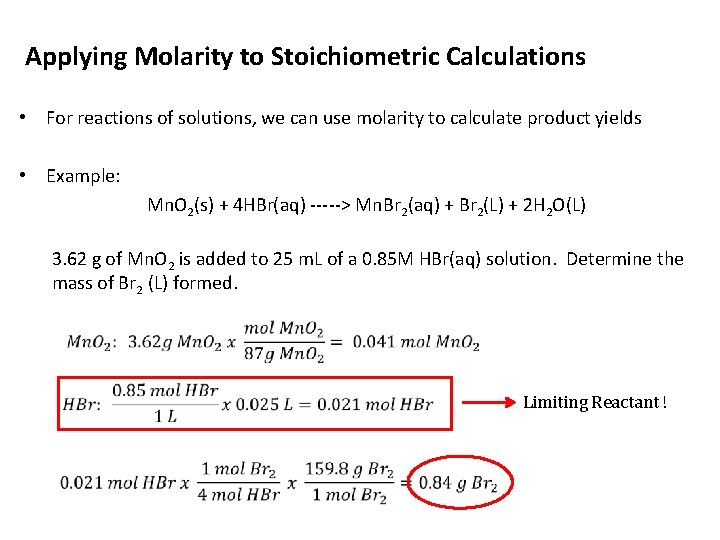
Applying Molarity to Stoichiometric Calculations • For reactions of solutions, we can use molarity to calculate product yields • Example: Mn. O 2(s) + 4 HBr(aq) -----> Mn. Br 2(aq) + Br 2(L) + 2 H 2 O(L) 3. 62 g of Mn. O 2 is added to 25 m. L of a 0. 85 M HBr(aq) solution. Determine the mass of Br 2 (L) formed. Limiting Reactant !
 Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Structural steel connection calculations calculations
Structural steel connection calculations calculations Reactions in aqueous solutions
Reactions in aqueous solutions Balancing redox reactions in basic solution
Balancing redox reactions in basic solution Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Aqueous solutions module
Aqueous solutions module Freezing point chapter 13
Freezing point chapter 13 General properties of aqueous solutions
General properties of aqueous solutions Reactions in aqueous solutions practice problems
Reactions in aqueous solutions practice problems Electrical conductivity of aqueous solutions
Electrical conductivity of aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Solubility guidelines for aqueous solutions
Solubility guidelines for aqueous solutions