Chargin with Coulomb l The SI unit of
Chargin’ with Coulomb
l The SI unit of charge is the Coulomb, C. For historical reasons, a charge of 1 C is the charge of 6. 24 billion (6. 24 x 1018) electrons. l Write this number without the use of scientific notation. l 6, 240, 000, 000 (that’s 16 zeroes)

l This might seem like a large number of electrons but it represents only the amount of charge which passes through a common 100 W bulb in about one SECOND.

l In 1785, CHARLES Coulomb developed Coulomb’s law which relates the amount of electrostatic force between CHARGES.
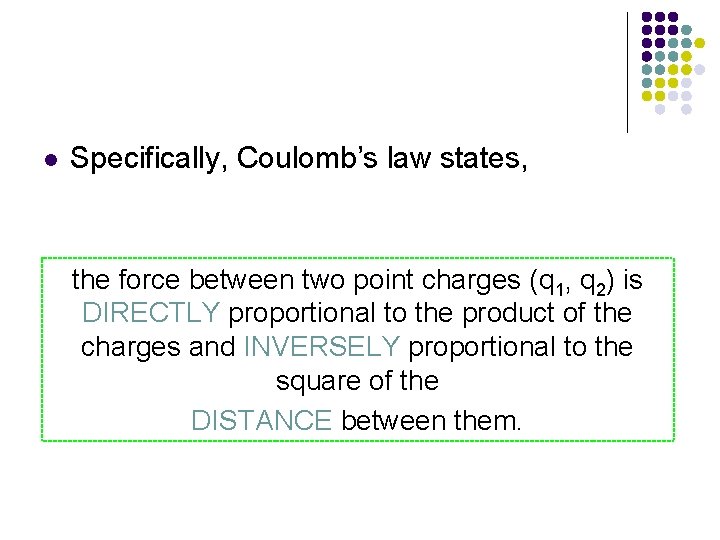
l Specifically, Coulomb’s law states, the force between two point charges (q 1, q 2) is DIRECTLY proportional to the product of the charges and INVERSELY proportional to the square of the DISTANCE between them.
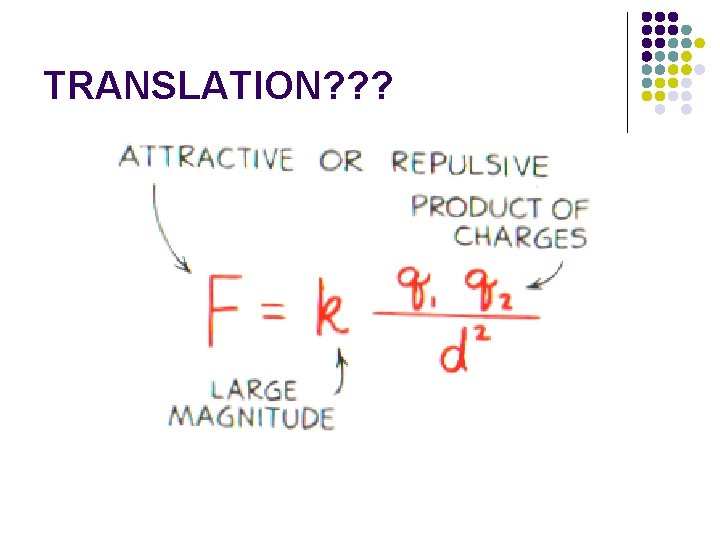
TRANSLATION? ? ?
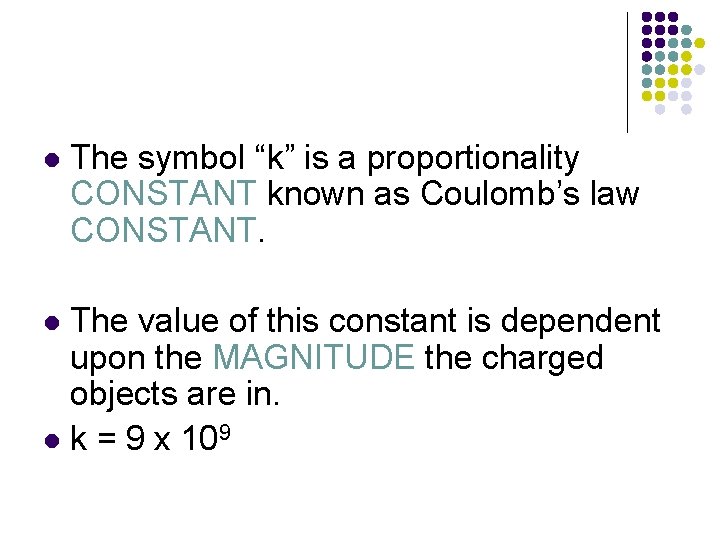
l The symbol “k” is a proportionality CONSTANT known as Coulomb’s law CONSTANT. The value of this constant is dependent upon the MAGNITUDE the charged objects are in. l k = 9 x 109 l
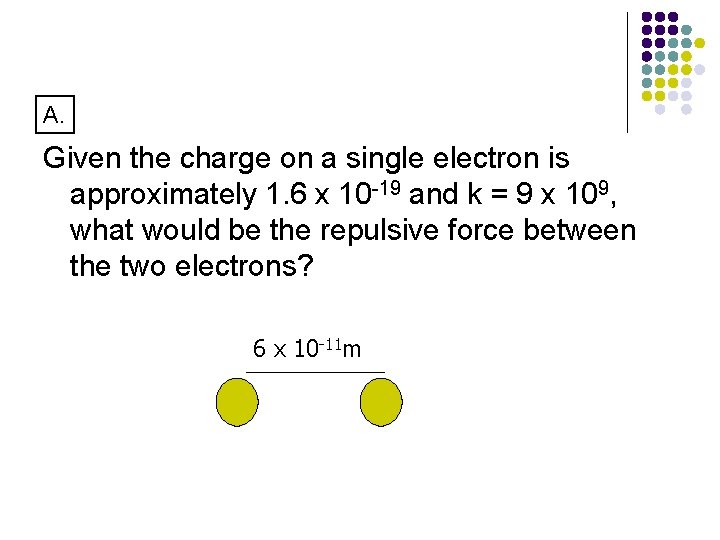
A. Given the charge on a single electron is approximately 1. 6 x 10 -19 and k = 9 x 109, what would be the repulsive force between the two electrons? 6 x 10 -11 m
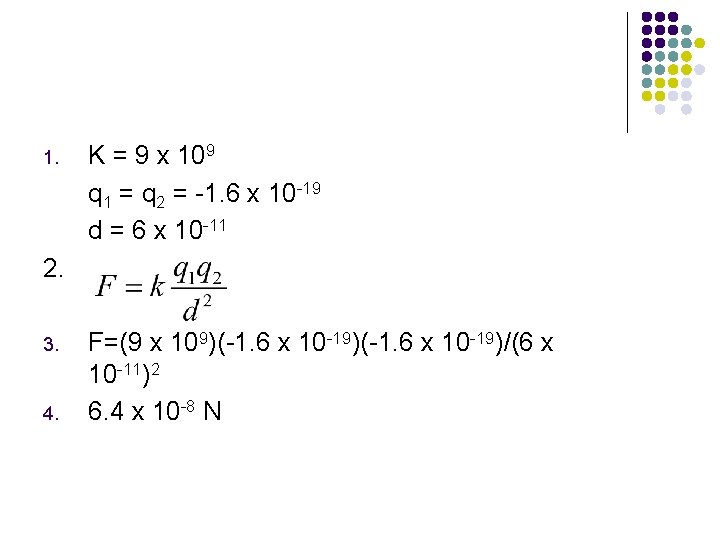
1. K = 9 x 109 q 1 = q 2 = -1. 6 x 10 -19 d = 6 x 10 -11 2. 3. 4. F=(9 x 109)(-1. 6 x 10 -19)/(6 x 10 -11)2 6. 4 x 10 -8 N

l HOMEWORK l l l pg 13 worksheet DUE TOMORROW 2/20
- Slides: 11