Chapter 8 Yonsei University Department of Biochemistry The

Chapter 8. 효소반응속도론 Yonsei University Department of Biochemistry The laboratory of tumorigenesis and senescence 서진호
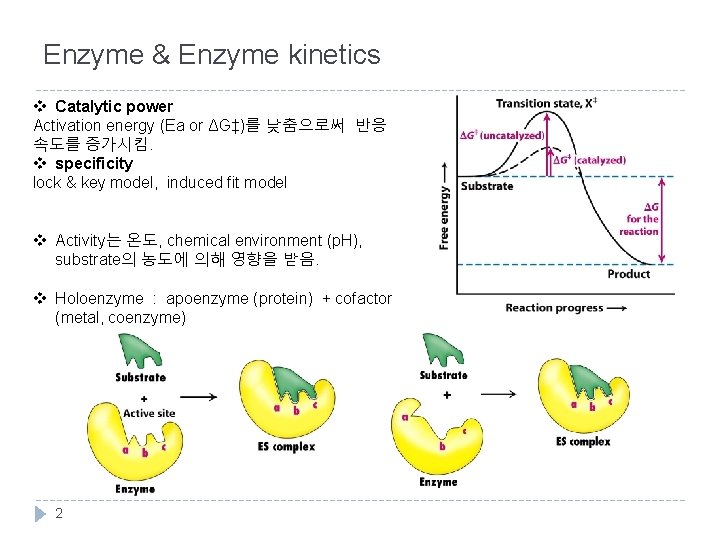
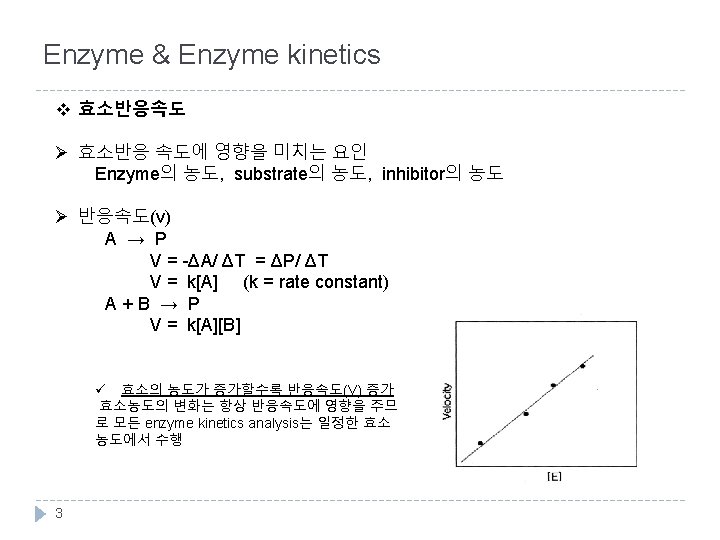
Enzyme & Enzyme kinetics v Catalytic power Activation energy (Ea or ΔG‡)를 낮춤으로써 반응 속도를 증가시킴. v specificity lock & key model, induced fit model v Activity는 온도, chemical environment (p. H), substrate의 농도에 의해 영향을 받음. v Holoenzyme : apoenzyme (protein) + cofactor (metal, coenzyme) 2
![Michaelis-Menten kinetics equation V 0 = Vmax [S] Km + [S] Vmax = maximum Michaelis-Menten kinetics equation V 0 = Vmax [S] Km + [S] Vmax = maximum](http://slidetodoc.com/presentation_image_h/1243524a4f93ffc4c310dbd299a82538/image-4.jpg)
Michaelis-Menten kinetics equation V 0 = Vmax [S] Km + [S] Vmax = maximum velocity V 0 = initial velocity [S] = substrate conc. Km = (k -1+k 2)/k 1 = Vmax /2 일 때 기질농도 * k 1 >> k-1 * steady-state assumption => [ES]는 일정 k 1[E][S] = (k -1+k 2)[ES] [E][S] / [ES] = (k -1+k 2)/k 1 4
![Double-reciprocal plot Vmax [S] V 0 = 1 V 0 Km + [S] = Double-reciprocal plot Vmax [S] V 0 = 1 V 0 Km + [S] =](http://slidetodoc.com/presentation_image_h/1243524a4f93ffc4c310dbd299a82538/image-5.jpg)
Double-reciprocal plot Vmax [S] V 0 = 1 V 0 Km + [S] = y = Km Vmax ax · 1 [S] + + Lineweaver-Burk plot Double-reciprocal plot 5 1 Vmax b
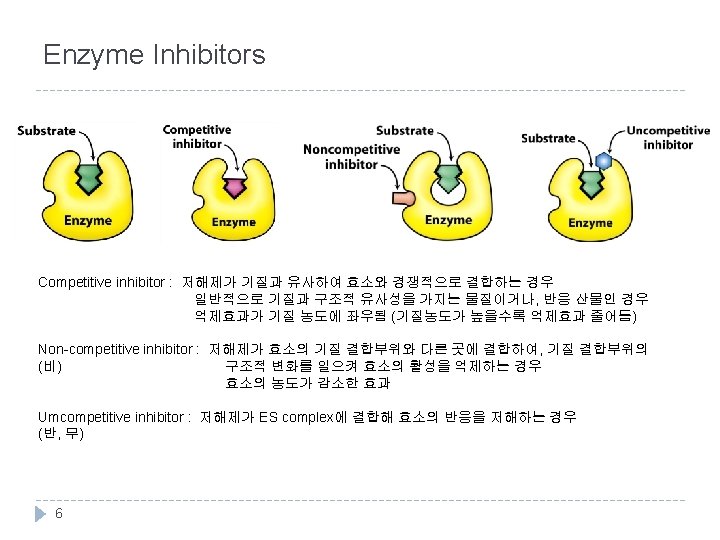
![Competitive Inhibitor Ki = [E][I]/[EI] KMAPP 증가 : x절편 증가 Vmax. APP 변함없음 : Competitive Inhibitor Ki = [E][I]/[EI] KMAPP 증가 : x절편 증가 Vmax. APP 변함없음 :](http://slidetodoc.com/presentation_image_h/1243524a4f93ffc4c310dbd299a82538/image-7.jpg)
Competitive Inhibitor Ki = [E][I]/[EI] KMAPP 증가 : x절편 증가 Vmax. APP 변함없음 : y절편 그대로 7
![Uncompetitive Inhibitor Ki = [ES][I]/[ESI] KMAPP 감소 : x절편 감소 Vmax. APP 감소 : Uncompetitive Inhibitor Ki = [ES][I]/[ESI] KMAPP 감소 : x절편 감소 Vmax. APP 감소 :](http://slidetodoc.com/presentation_image_h/1243524a4f93ffc4c310dbd299a82538/image-8.jpg)
Uncompetitive Inhibitor Ki = [ES][I]/[ESI] KMAPP 감소 : x절편 감소 Vmax. APP 감소 : y절편 증가 8
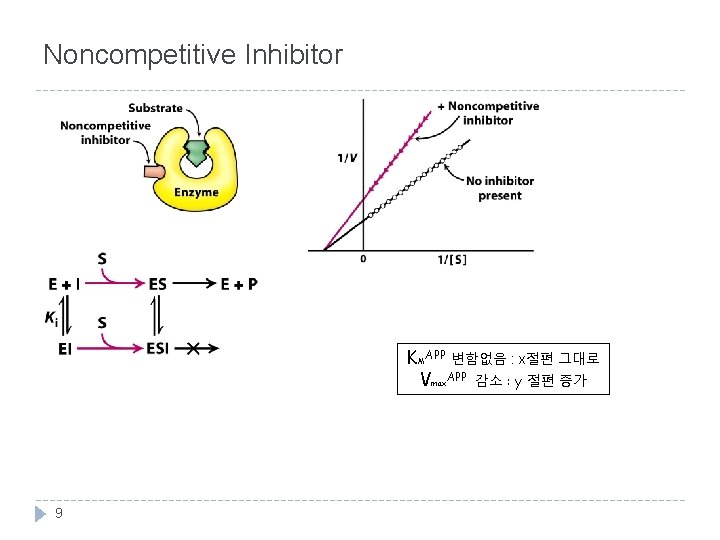
Noncompetitive Inhibitor KMAPP 변함없음 : x절편 그대로 Vmax. APP 감소 : y 절편 증가 9
![Double-reciprocal plot for Inhibitor KMAPP= KM(1 + [I]/Ki) Vmax. APP = Vmax /(1 + Double-reciprocal plot for Inhibitor KMAPP= KM(1 + [I]/Ki) Vmax. APP = Vmax /(1 +](http://slidetodoc.com/presentation_image_h/1243524a4f93ffc4c310dbd299a82538/image-10.jpg)
Double-reciprocal plot for Inhibitor KMAPP= KM(1 + [I]/Ki) Vmax. APP = Vmax /(1 + [I]/Ki) 10
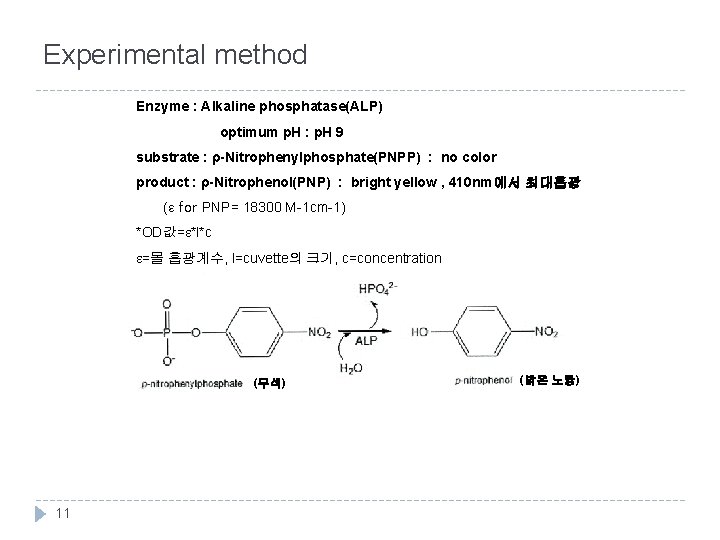
Experimental method Enzyme : Alkaline phosphatase(ALP) optimum p. H : p. H 9 substrate : ρ-Nitrophenylphosphate(PNPP) : no color product : ρ-Nitrophenol(PNP) : bright yellow , 410 nm에서 최대흡광 (ε for PNP= 18300 M-1 cm-1) *OD값=e*l*c e=몰 흡광계수, l=cuvette의 크기, c=concentration (무색) 11 (밝은 노랑)
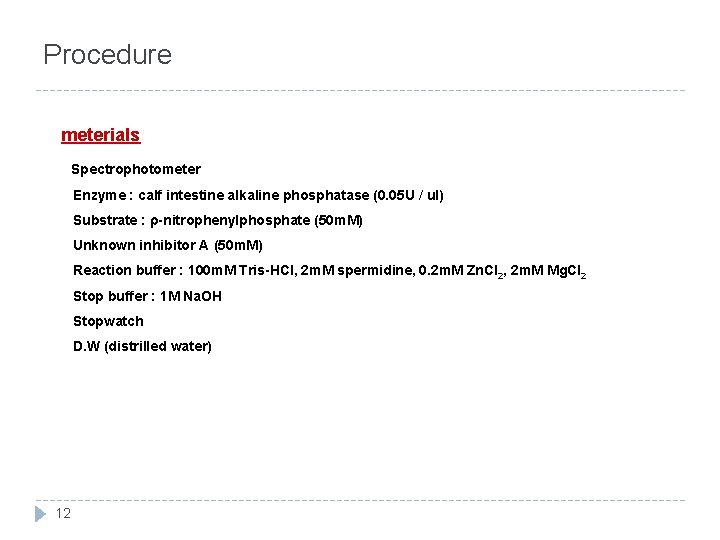
Procedure meterials Spectrophotometer Enzyme : calf intestine alkaline phosphatase (0. 05 U / ul) Substrate : ρ-nitrophenylphosphate (50 m. M) Unknown inhibitor A (50 m. M) Reaction buffer : 100 m. M Tris-HCl, 2 m. M spermidine, 0. 2 m. M Zn. Cl 2, 2 m. M Mg. Cl 2 Stop buffer : 1 M Na. OH Stopwatch D. W (distrilled water) 12

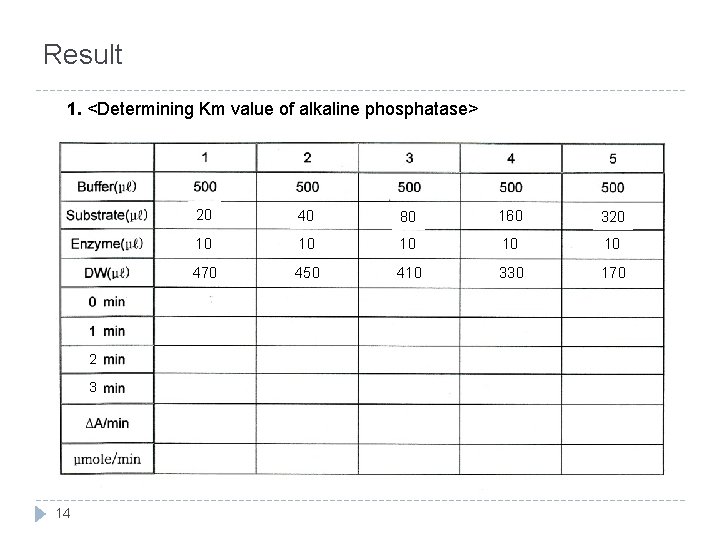
Result 1. <Determining Km value of alkaline phosphatase> 2 3 14 20 40 80 160 320 10 10 10 470 450 410 330 170
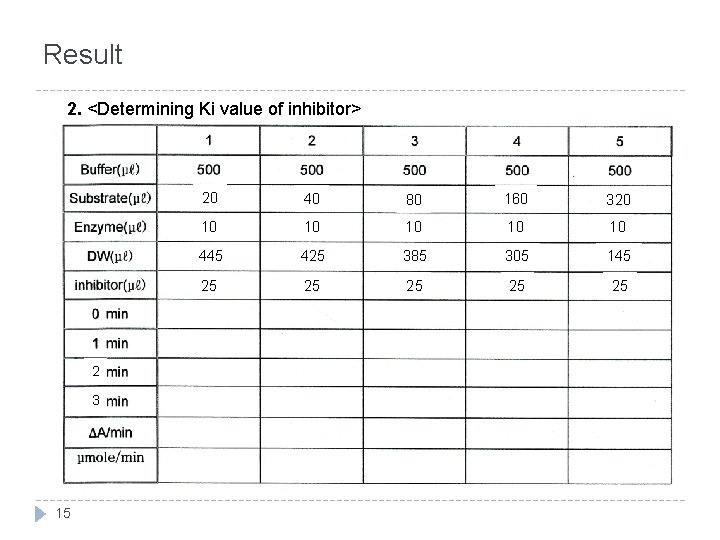
Result 2. <Determining Ki value of inhibitor> 2 3 15 20 40 80 160 320 10 10 10 445 425 385 305 145 25 25 25


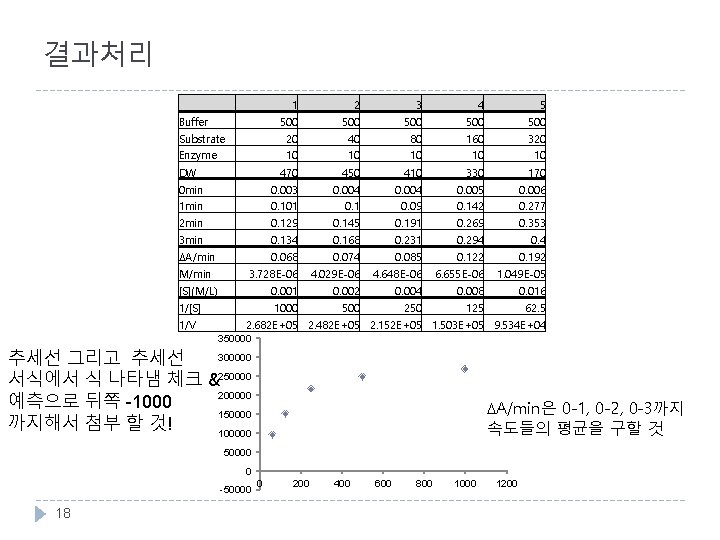
결과처리 1 2 3 4 5 500 500 500 20 10 40 10 80 10 160 10 320 10 470 450 410 330 170 0 min 1 min 2 min 0. 003 0. 101 0. 129 0. 004 0. 145 0. 004 0. 09 0. 191 0. 005 0. 142 0. 269 0. 006 0. 277 0. 353 3 min 0. 134 0. 168 0. 231 0. 294 0. 4 DA/min M/min 0. 068 3. 728 E-06 0. 074 4. 029 E-06 0. 085 4. 648 E-06 0. 122 6. 655 E-06 0. 192 1. 049 E-05 [S](M/L) 0. 001 0. 002 0. 004 0. 008 0. 016 Buffer Substrate Enzyme DW 1/[S] 1/V 1000 500 250 125 62. 5 2. 682 E+05 2. 482 E+05 2. 152 E+05 1. 503 E+05 9. 534 E+04 350000 300000 추세선 그리고 추세선 서식에서 식 나타냄 체크 &250000 200000 예측으로 뒤쪽 -1000 150000 까지해서 첨부 할 것! 100000 DA/min은 0 -1, 0 -2, 0 -3까지 속도들의 평균을 구할 것 50000 0 -50000 18 0 200 400 600 800 1000 1200
- Slides: 18