CHAPTER 6 TITRIMETRIC METHODS OF ANALYSIS Titrimetric methods










- Slides: 18

CHAPTER 6 TITRIMETRIC METHODS OF ANALYSIS

Titrimetric methods include a large and powerful group of quantitative procedures based on measuring the amount of a reagent of known concentration (standard solutiontitrant) that is consumed by the analyte. Titrimetry is a term which includes a group of analytical methods based on determining the quantity of a reagent of known concentration that is required to react completely with the analyte.

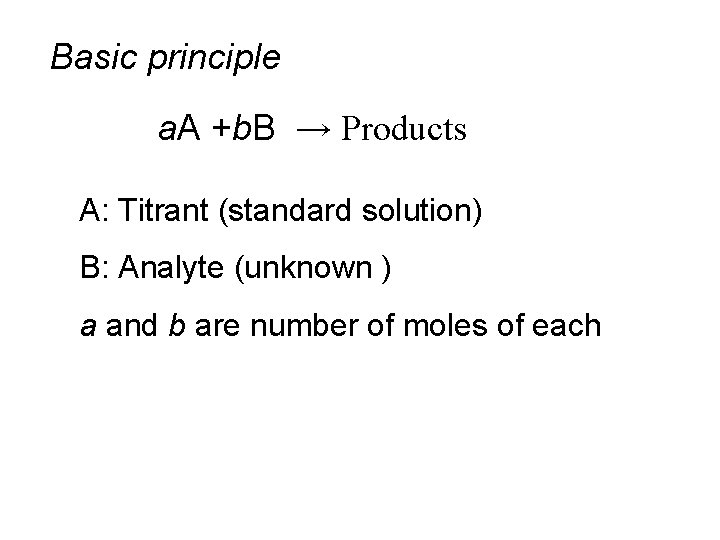
Basic principle a. A +b. B → Products A: Titrant (standard solution) B: Analyte (unknown ) a and b are number of moles of each

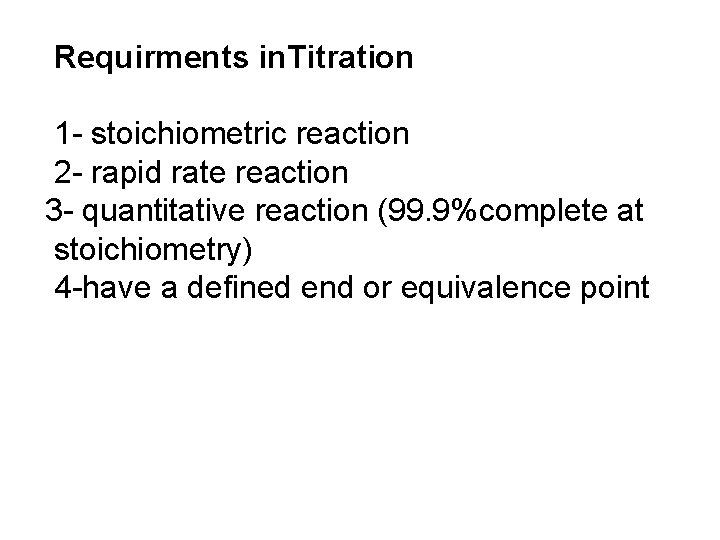
Requirments in. Titration 1 - stoichiometric reaction 2 - rapid rate reaction 3 - quantitative reaction (99. 9%complete at stoichiometry) 4 -have a defined end or equivalence point

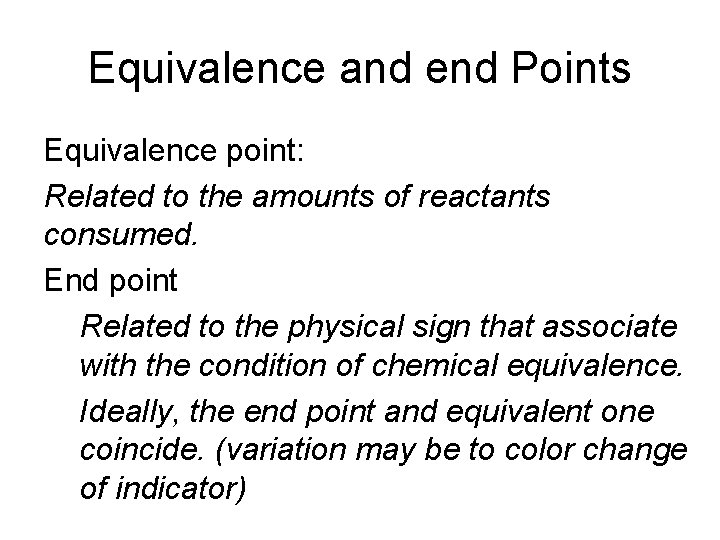
Equivalence and end Points Equivalence point: Related to the amounts of reactants consumed. End point Related to the physical sign that associate with the condition of chemical equivalence. Ideally, the end point and equivalent one coincide. (variation may be to color change of indicator)

Standard solutions Standard Solution - A primary standard - A secondary standard Standardization A process in which concentration of a volumetric solution is determined by titrating it with a known mass of a primary standard.

§ Titration: This is performed by adding a standard solution from a buret or other liquid- dispensing device to a solution of the analyte until the point at which the reaction is believed to be complete. Solution of Na. OH Solution of HCl 5 m. L

PRIMARY STANDARD • • • HIGH PURITY ATMOSPHERIC STABILITY INDEPENDENT OF HUMIDITY MODEST COST LARGE MOLAR MASS NON HGYROSCOPIC

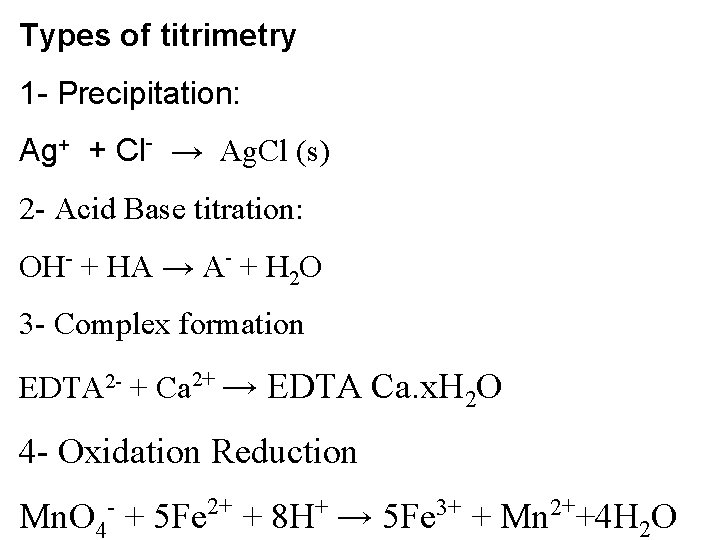
Types of titrimetry 1 - Precipitation: Ag+ + Cl- → Ag. Cl (s) 2 - Acid Base titration: OH- + HA → A- + H 2 O 3 - Complex formation EDTA 2 - + Ca 2+ → EDTA Ca. x. H 2 O 4 - Oxidation Reduction Mn. O 4 - + 5 Fe 2+ + 8 H+ → 5 Fe 3+ + Mn 2++4 H 2 O

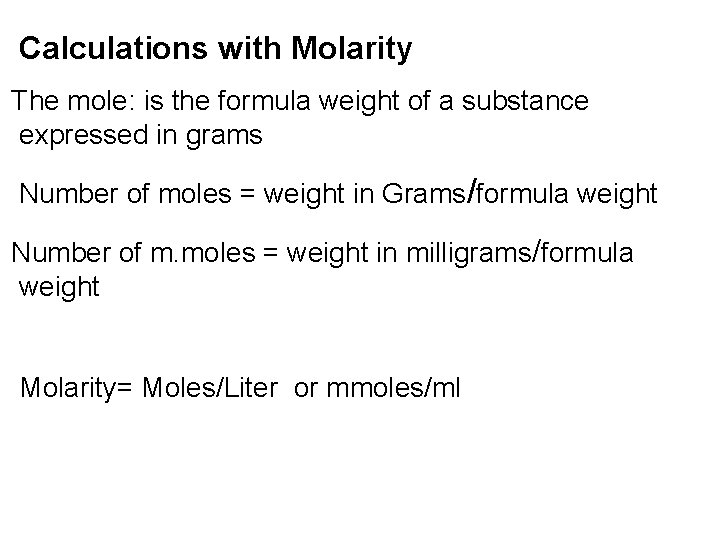
Calculations with Molarity The mole: is the formula weight of a substance expressed in grams Number of moles = weight in Grams/formula weight Number of m. moles = weight in milligrams/formula weight Molarity= Moles/Liter or mmoles/ml

Moles= Volume (L). (M) Weight (g) = Volume (L). (M). form weight (g) m. Moles= Volume (ml). (M) Weight (mg) = Volume (ml). (M). form weight (g)

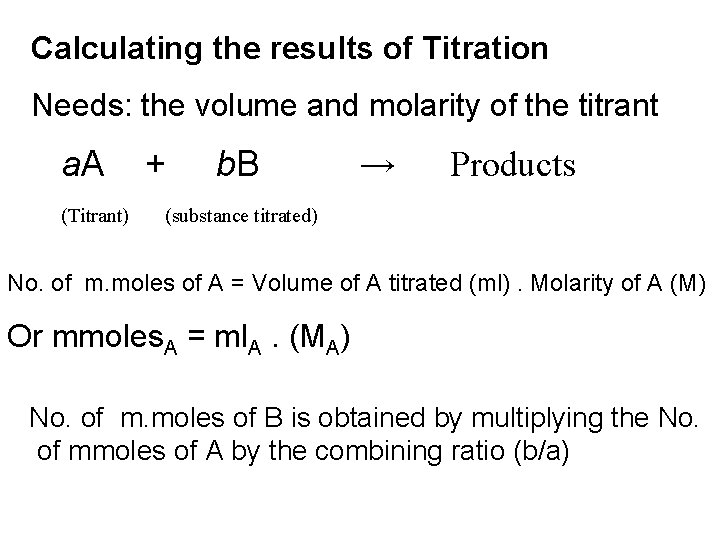
Calculating the results of Titration Needs: the volume and molarity of the titrant a. A + b. B → (Titrant) Products (substance titrated) No. of m. moles of A = Volume of A titrated (ml). Molarity of A (M) Or mmoles. A = ml. A. (MA) No. of m. moles of B is obtained by multiplying the No. of mmoles of A by the combining ratio (b/a)

mmoles. B = mmoles. A. (b/a) = ml. A. (MA). (b/a)

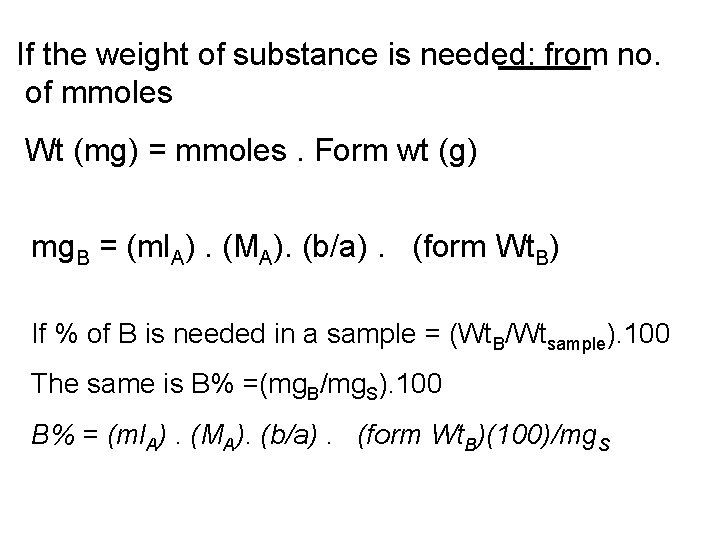
If the weight of substance is needed: from no. of mmoles Wt (mg) = mmoles. Form wt (g) mg. B = (ml. A). (MA). (b/a). (form Wt. B) If % of B is needed in a sample = (Wt. B/Wtsample). 100 The same is B% =(mg. B/mg. S). 100 B% = (ml. A). (MA). (b/a). (form Wt. B)(100)/mg. S


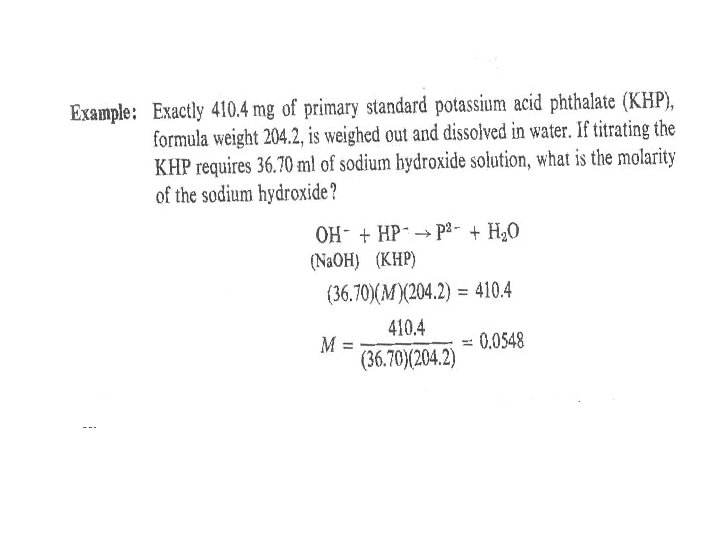
Calculating the molarity of a solution from a standardizing titration This means that from the weight of primary standard substance (B )when dissolved and titrated with other solution, the molarity of the solution (A) can be calculated as follows: a. A + b. B → (Solution. A) Products (primary standard) (ml. A) (MA)/a = (ml. B) (MB)/b (ml. B) (MB) = mmoles. B = mg. B /Form Wt. B (MA)/a = mg. B/ (ml. A) (b/a) (Form Wt. B)


Exercises Excercize 1 How many ml of 0. 25 M Na. OH will react with 10. 0 ml of 0. 10 M H 2 SO 4.