Chapter 5 1 Announcements Homework 5 1 due







- Slides: 30

Chapter 5. 1 Announcements: Homework 5. 1: due Thursday, March 21, in class long homework start early Exercises: 1, 2, 3, 4, 6, 7, 8, 12, 13, 15, 16, 18 Problems: 1, 2, 3, 4, 6, 7, 8, 9 - All grades will continue to be posted at: http: //www. wfu. edu/~gutholdm/Physics 110/phy 110. htm - Listed by last four digits of student ID - We’ll now cover only parts of each chapter (tentative outline): - 5. 1 Balloons - 11. 1 Household Magnets & Electric Motor - 7. 1 Woodstoves - 11. 2 Electric Power Distribution - 9. 1 Clocks - 15. 1. Optics, cameras, lenses - 9. 2 Musical Instruments - 16. 1 Nuclear Weapons - 10. 3 Flashlights

Chapter 5. 1: Balloons (long Chapter section with many concepts Demos and Objects - helium balloons imploding can Magdeburg spheres expanding foam particles in a box submerging metal block weighing things in water diet coke and real coke Concepts - Buoyancy - Archimedes’ principle - air and air pressure - density - pressure - temperature - earth’s atmosphere - helium balloons - hot air balloons - ideal gas law

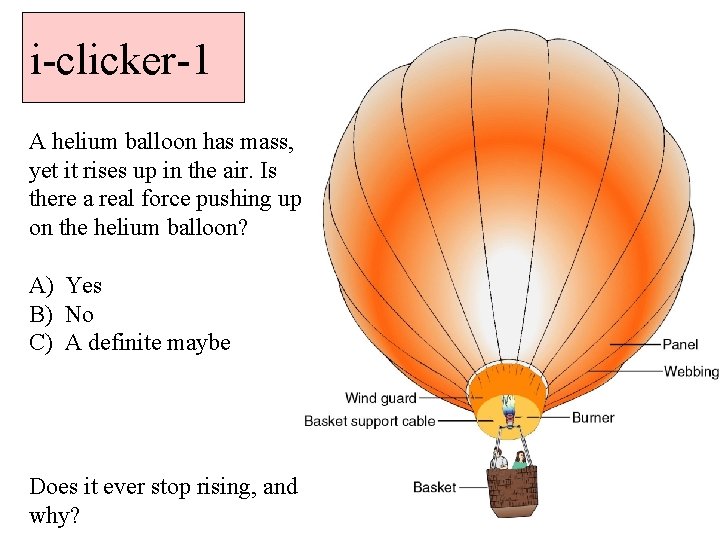
i-clicker-1 A helium balloon has mass, yet it rises up in the air. Is there a real force pushing up on the helium balloon? A) Yes B) No C) A definite maybe Does it ever stop rising, and why?

Air and Air Pressure What is air? • Air is a gas – Consists of individual atoms and molecules – Particles kept separate by their thermal energy – Particles bounce around in free fall – Air does have a mass – Air does have weight – 1 kg of air can have many different shapes – Air is compressible

Air and Air Pressure • Air has pressure – – – Air particles bounce around The higher the temperature, the more they bounce Air particles exerts forces on container walls Average force is proportional to surface area Average force per unit area is called “pressure” This formula is true for many situations. Unit of pressure: 1 N/m 2 = 1 Pascal = 1 Pa Blaise Pascal (June 19, 1623, in Clermont-Ferrand, France – August 19, 1662, in Paris) was a French mathematician, physicist, and religious philosopher. He was a child prodigy who was educated by his father. Pascal's earliest work was in the natural and applied sciences where he made important contributions to the construction of mechanical calculators, the study of fluids, and clarified the concepts of pressure and vacuum by generalizing the work of Evangelista Torricelli

Examples: You weigh 800 N, you fell like wearing high heels (area 1 cm 2 = 0. 0001 m 2) and you step on somebody’s foot. What pressure does that person feel? A tank weighs 400, 000 N and rolls around on a 10 m 2 chain. What is the pressure on the ground?

Pressure Imbalances • Balanced pressure exerts no overall force – Forces on opposite sides of object cancel • Unbalanced pressure exerts an overall force – Forces on opposites sides of object don’t cancel – Forces push object toward lower pressure

i-clicker-2 (Madgeburg spheres) In 1654, Otto von Guericke gave the citizens of Magdeburg a remarkable lesson in the force of the atmospheric pressure. He machined two hollow hemispheres, twenty inches in diameter (0. 5 m) so they fit snuggly into a sealed sphere. He pumped the air out of it. Then he put sixteen horses, eight on each side, to the task of pulling the halves apart. The horses hard a very hard time pulling them apart. If the atmospheric pressure is 1. 0· 105 Pa, what force would be required to pull the spheres apart? A. 5000 N B. 10, 000 N C. 20, 000 N D. 50, 000 N E. 100, 000 N

You purchase a bottle of water in an airplane, drink half of it and then seal it again. When you arrive at the airport, the bottle is all dented in. Explain.

Air and Density What is density? • Air has density – Air particles have mass – Each volume of air has a certain amount of mass – Average mass per unit volume is called “density” Units: kg/m 3

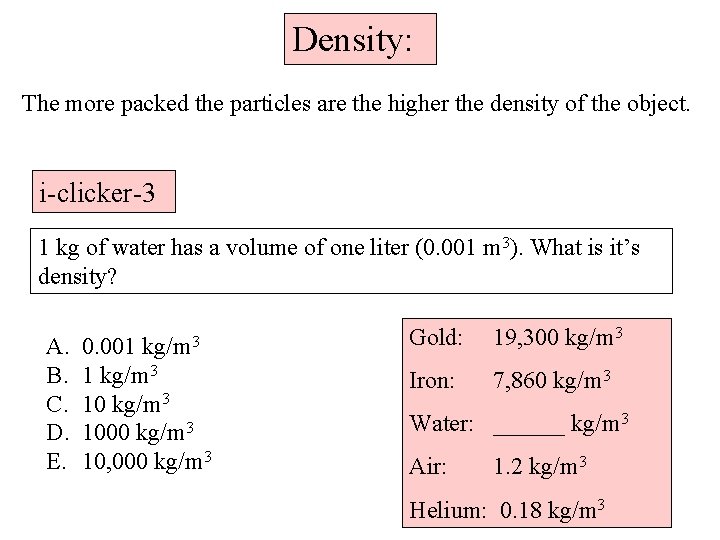
Density: The more packed the particles are the higher the density of the object. i-clicker-3 1 kg of water has a volume of one liter (0. 001 m 3). What is it’s density? A. B. C. D. E. kg/m 3 0. 001 1 kg/m 3 1000 kg/m 3 10, 000 kg/m 3 Gold: 19, 300 kg/m 3 Iron: 7, 860 kg/m 3 Water: ______ kg/m 3 Air: 1. 2 kg/m 3 Helium: 0. 18 kg/m 3

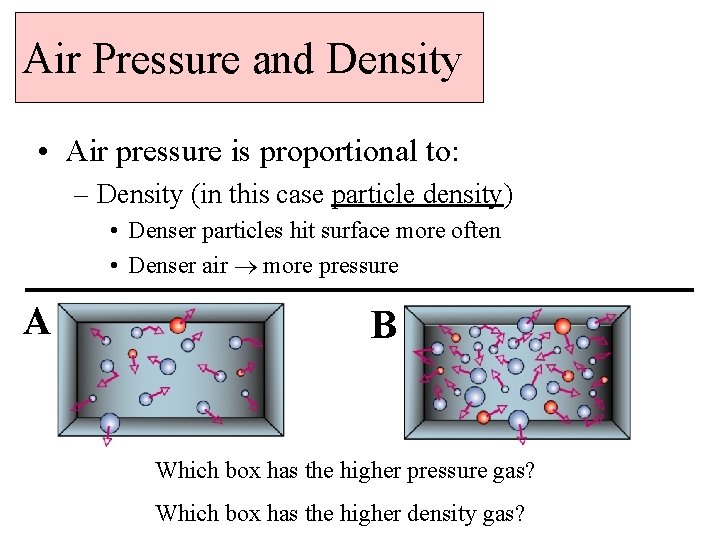
Air Pressure and Density • Air pressure is proportional to: – Density (in this case particle density) • Denser particles hit surface more often • Denser air more pressure A B Which box has the higher pressure gas? Which box has the higher density gas?

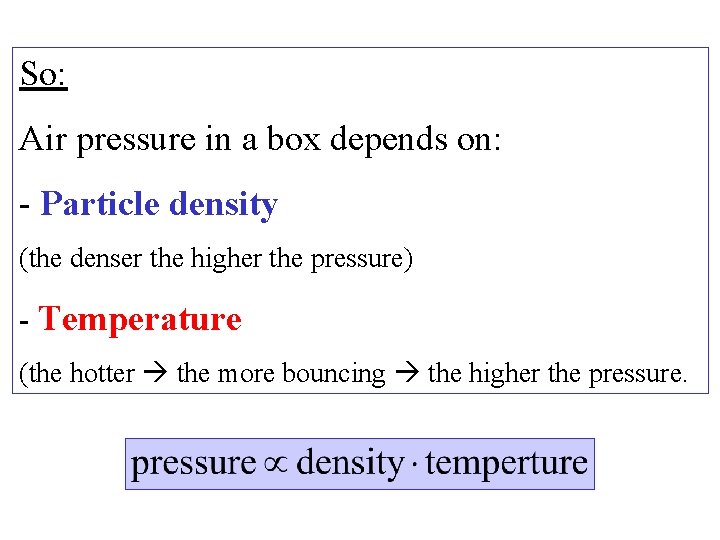
So: Air pressure in a box depends on: - Particle density (the denser the higher the pressure) - Temperature (the hotter the more bouncing the higher the pressure.

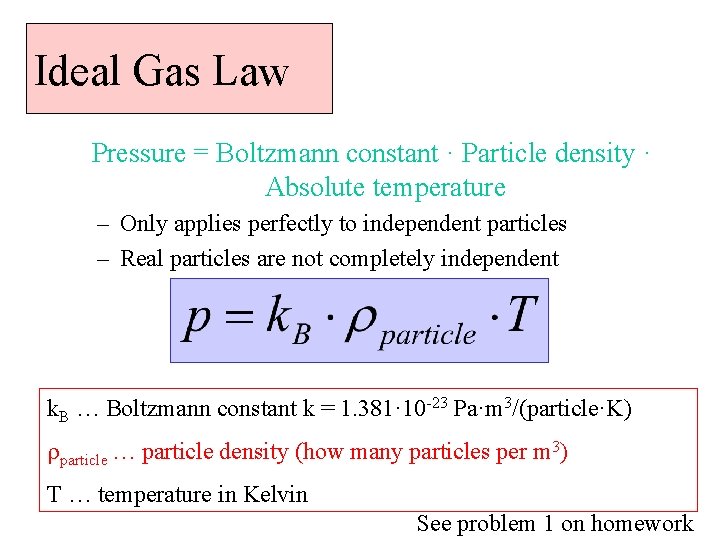
Ideal Gas Law Pressure = Boltzmann constant · Particle density · Absolute temperature – Only applies perfectly to independent particles – Real particles are not completely independent k. B … Boltzmann constant k = 1. 381· 10 -23 Pa·m 3/(particle·K) rparticle … particle density (how many particles per m 3) T … temperature in Kelvin See problem 1 on homework

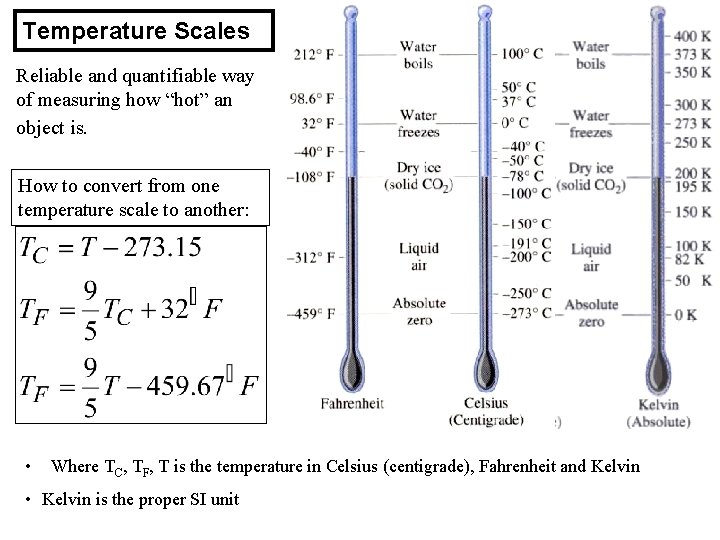
An Aside About Temperature • Air has temperature – Air particles have thermal kinetic energy – Average thermal kinetic energy is proportional to absolute temperature • SI absolute temperature scale: kelvins or K – 0 K is absolute zero—no thermal energy left – 1 K is equivalent to 1 degree Celsius – room temperature ~ 293 K = 20°C = 68 F – 0 K = - 273. 15°C = - 459. 67 F

Temperature Scales Reliable and quantifiable way of measuring how “hot” an object is. How to convert from one temperature scale to another: • Where TC, TF, T is the temperature in Celsius (centigrade), Fahrenheit and Kelvin • Kelvin is the proper SI unit

Homework, Problem 1: The particle density of standard atmospheric air at 273. 15 K (0°C, 32 F) is 2. 687· 1025 particles/m 3. Using the ideal gas law, calculate the pressure of this air.

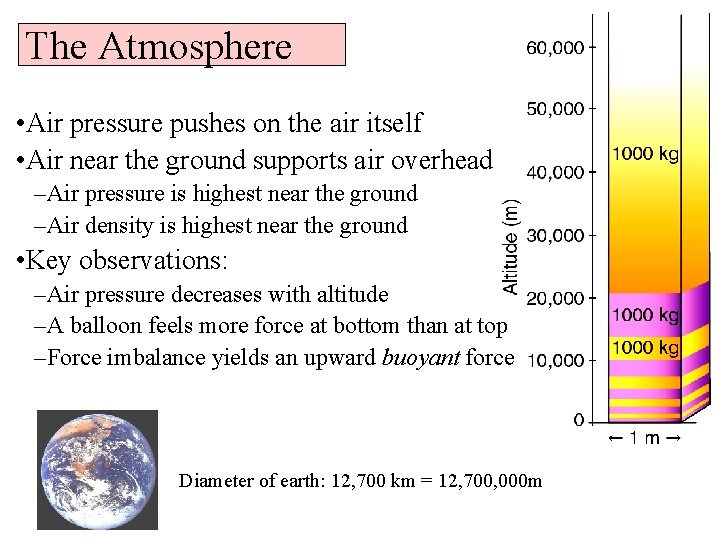
The Atmosphere • Air pressure pushes on the air itself • Air near the ground supports air overhead –Air pressure is highest near the ground –Air density is highest near the ground • Key observations: –Air pressure decreases with altitude –A balloon feels more force at bottom than at top –Force imbalance yields an upward buoyant force Diameter of earth: 12, 700 km = 12, 700, 000 m

Pressure Imbalances • Balanced pressure exerts no overall force – Forces on opposite sides of balloon cancel. • Unbalanced pressure exerts an overall force – Forces on top and bottom of balloon don’t cancel. – Forces push balloon toward lower pressure.

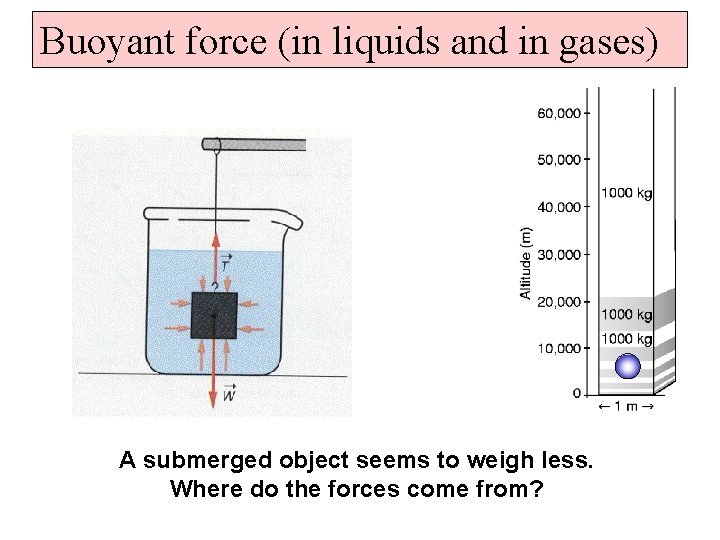
Buoyant force (in liquids and in gases) A submerged object seems to weigh less. Where do the forces come from?

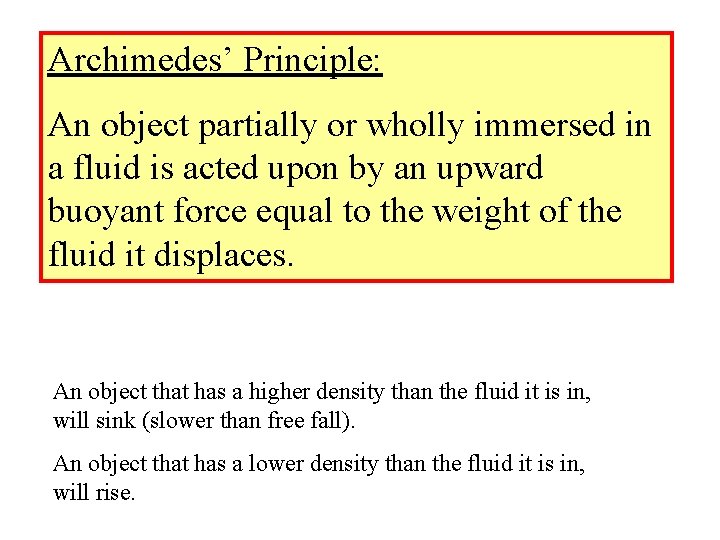
Archimedes’ Principle: An object partially or wholly immersed in a fluid is acted upon by an upward buoyant force equal to the weight of the fluid it displaces. An object that has a higher density than the fluid it is in, will sink (slower than free fall). An object that has a lower density than the fluid it is in, will rise.

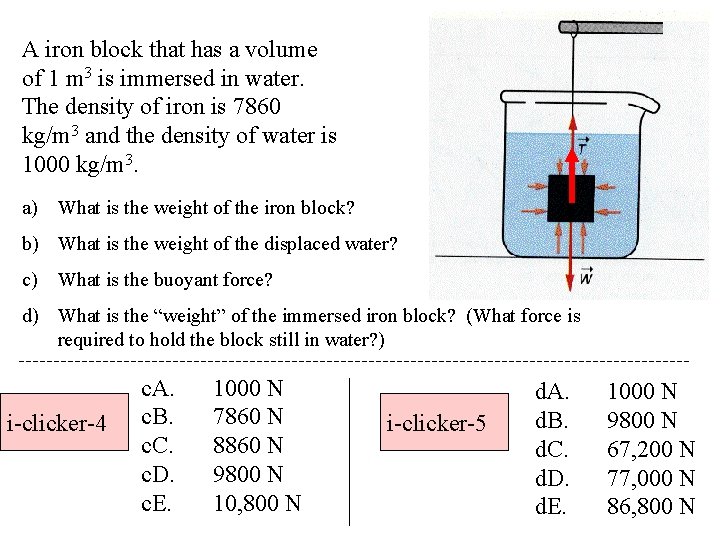
A iron block that has a volume of 1 m 3 is immersed in water. The density of iron is 7860 kg/m 3 and the density of water is 1000 kg/m 3. a) What is the weight of the iron block? b) What is the weight of the displaced water? c) What is the buoyant force? d) What is the “weight” of the immersed iron block? (What force is required to hold the block still in water? ) i-clicker-4 c. A. c. B. c. C. c. D. c. E. 1000 N 7860 N 8860 N 9800 N 10, 800 N i-clicker-5 d. A. d. B. d. C. d. D. d. E. 1000 N 9800 N 67, 200 N 77, 000 N 86, 800 N

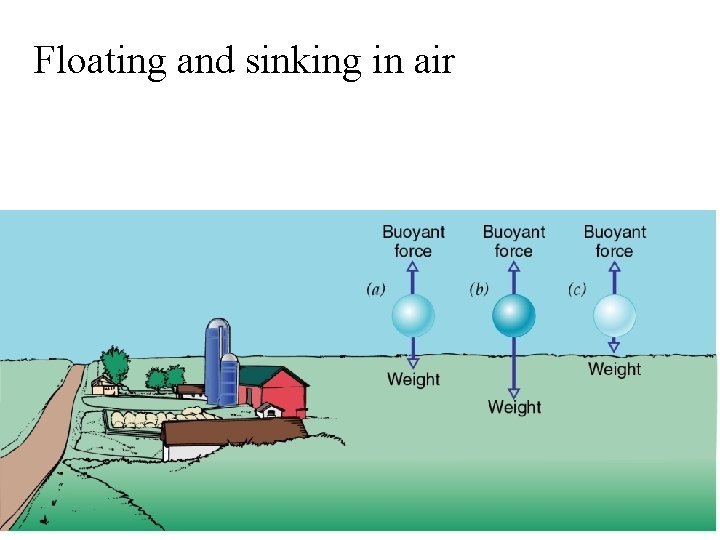
Floating and sinking in air

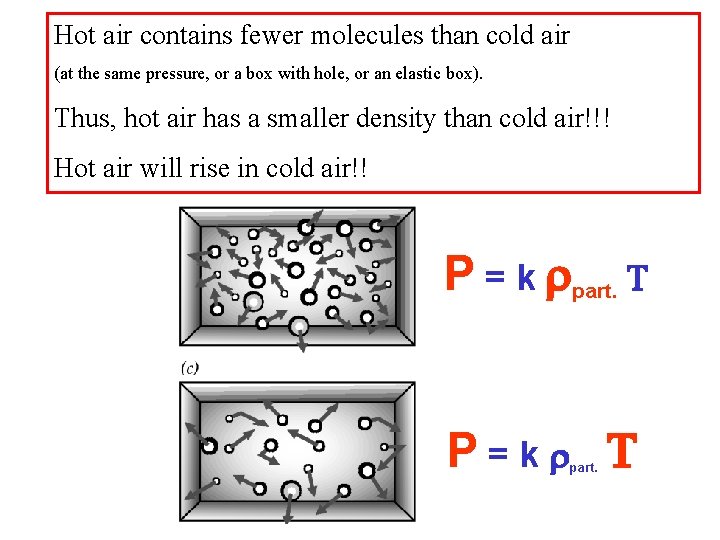
Hot air contains fewer molecules than cold air (at the same pressure, or a box with hole, or an elastic box). Thus, hot air has a smaller density than cold air!!! Hot air will rise in cold air!! P = k rpart. T P=kr T part.

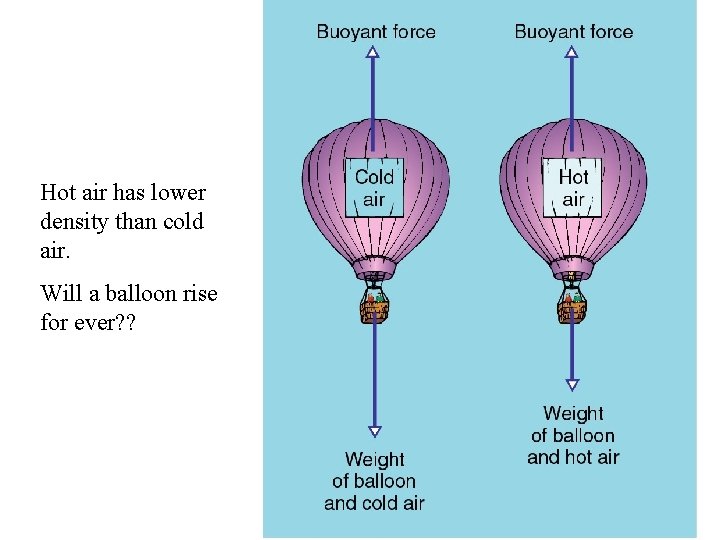
Hot air has lower density than cold air. Will a balloon rise for ever? ?



Hot-Air Balloon in Air • A rubber, hot-air-filled balloon – contains fewer air particles than if it were cold – weighs less than the cold air it displaces – experiences an upward net force in cold air – floats in cold air – has an average density less than that of cold air

Helium vs. Air • Replacing air particles with helium atoms – reduces the gas’s density • helium atoms have less mass than air particles

A helium balloon has a volume of 4000 m 3. The density of air is 1. 2 kg/m 3 and the density of helium is 0. 18 kg/m 3. a) What is the weight of the displaced air? b) What is the buoyant force on the balloon? c) What is the weight of the helium in the balloon? d) How much weight could the balloon carry up? i-clicker-6 (question d) d. A. d. B. d. C. d. D. d. E. 7, 000 N 40, 000 N 47, 000 N 54, 000 N None of the above