chapter 2 Quantitative Spectroscopy Light Properties Spectrophotometer Model


 Absorbance Transmittance(%) ε(L/mole-cm) L(cm) 30 2000 1. 00 3. Complete the following table. [X](M) Absorbance Transmittance(%) ε(L/mole-cm) L(cm) 30 2000 1. 00](https://slidetodoc.com/presentation_image/2a48a965404c03efb838916f306fd8c5/image-22.jpg)


- Slides: 31

chapter 2 Quantitative

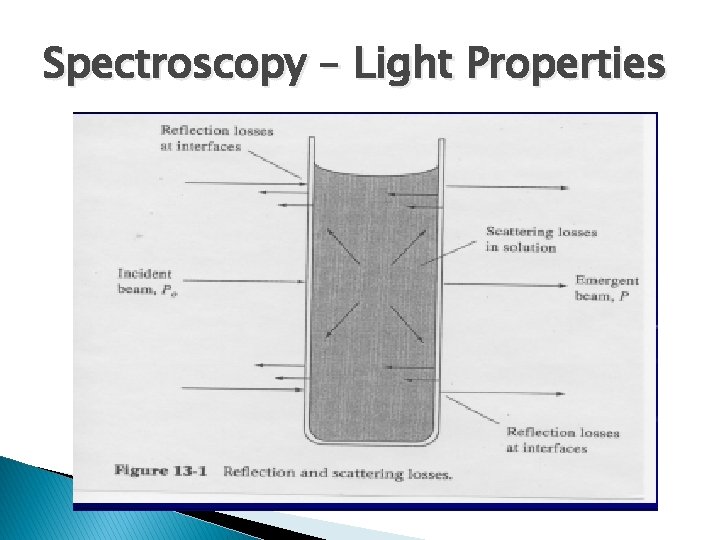
Spectroscopy – Light Properties

Spectrophotometer Model of light interaction with matrix and how spectrophotometer works

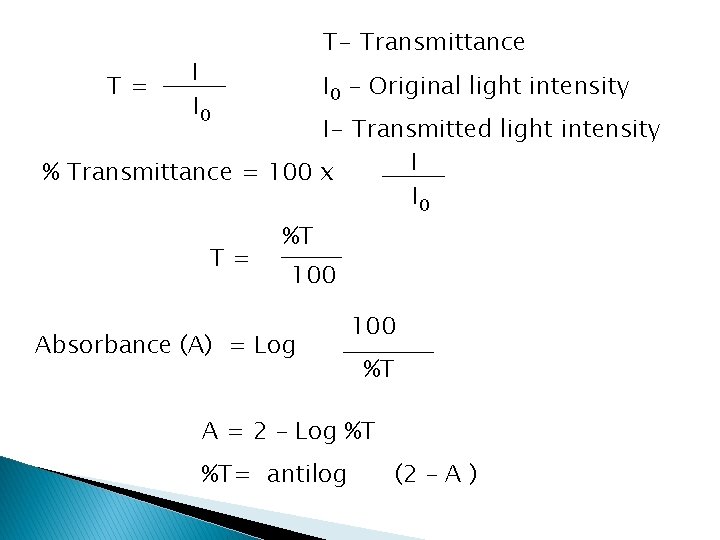
Spectroscopy – Quantification Terms Transmittance (T) – the fraction of incident radiation transmitted by the medium: T = I/I 0 Absorbance (A) – the amount of incident radiation absorb by the medium and expressed by: � A = log(1/T) = - log. T = log P 0/P

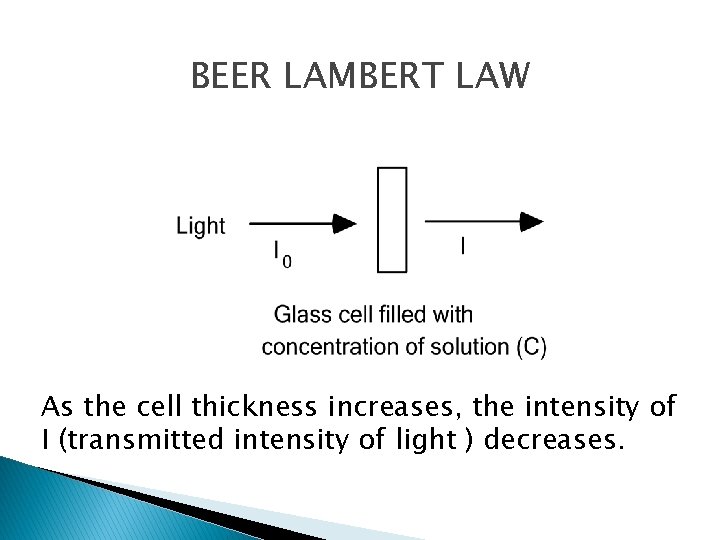
BEER LAMBERT LAW As the cell thickness increases, the intensity of I (transmitted intensity of light ) decreases.

T= T- Transmittance I I 0 - Original light intensity I- Transmitted light intensity I % Transmittance = 100 x I 0 T= %T 100 Absorbance (A) = Log 100 %T A = 2 – Log %T %T= antilog (2 – A )

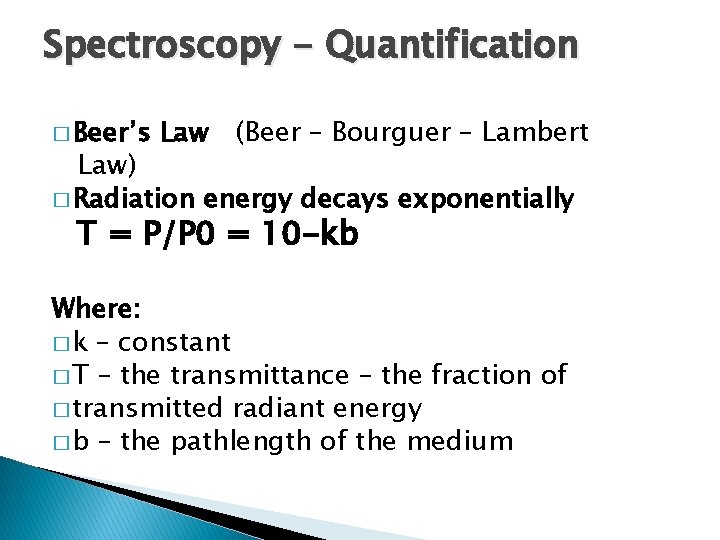
Spectroscopy - Quantification � Beer’s Law (Beer – Bourguer – Lambert Law) � Radiation energy decays exponentially T = P/P 0 = 10 -kb Where: � k – constant � T – the transmittance – the fraction of � transmitted radiant energy � b – the pathlength of the medium

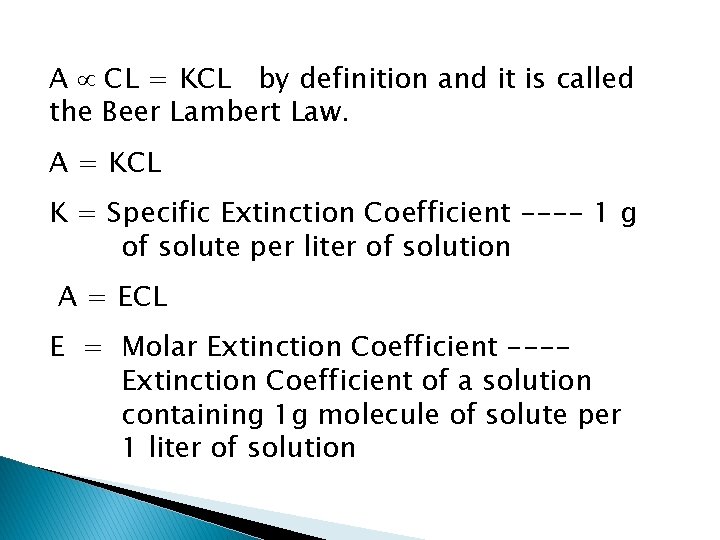
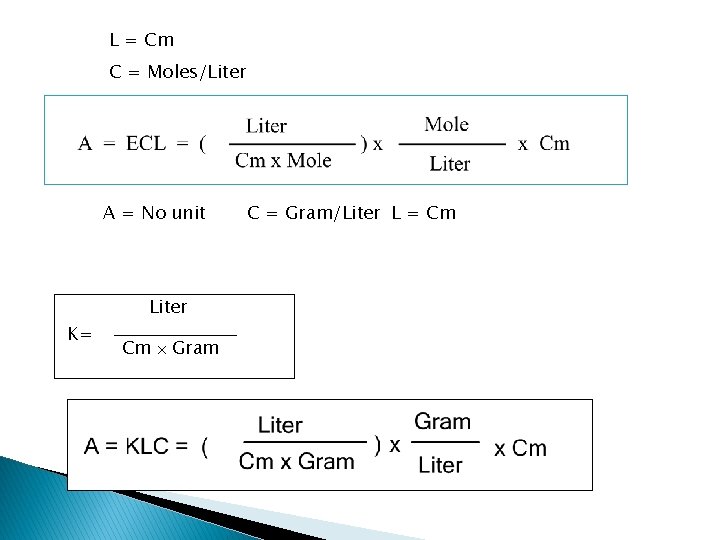
A CL = KCL by definition and it is called the Beer Lambert Law. A = KCL K = Specific Extinction Coefficient ---- 1 g of solute per liter of solution A = ECL E = Molar Extinction Coefficient ---Extinction Coefficient of a solution containing 1 g molecule of solute per 1 liter of solution

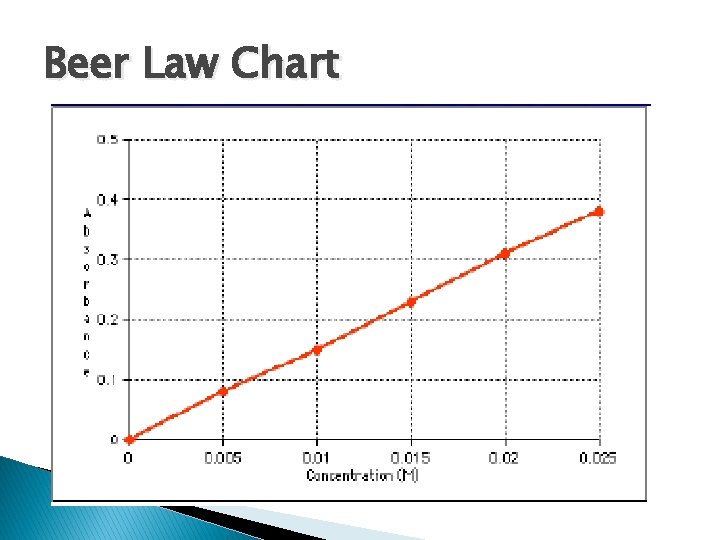
Beer Law Chart

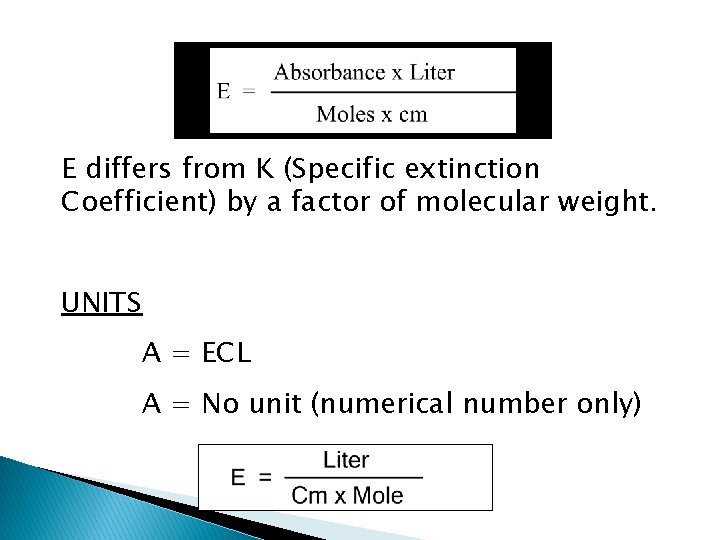
E differs from K (Specific extinction Coefficient) by a factor of molecular weight. UNITS A = ECL A = No unit (numerical number only)

L = Cm C = Moles/Liter A = KCL A = No unit K= Liter Cm Gram C = Gram/Liter L = Cm

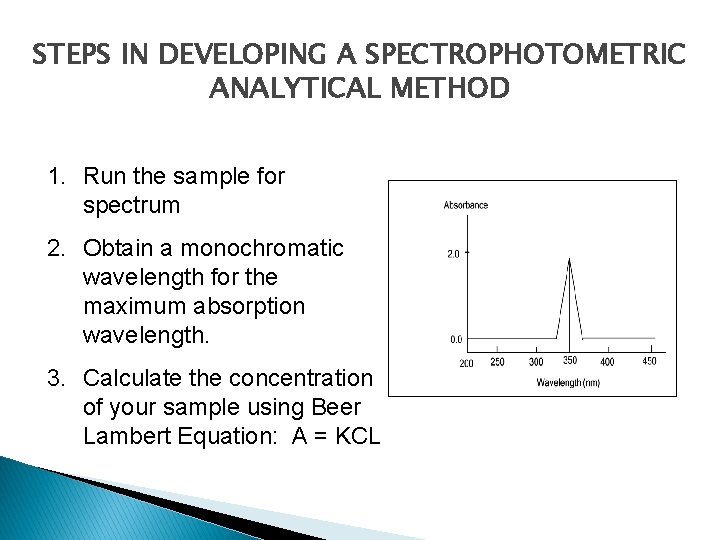
STEPS IN DEVELOPING A SPECTROPHOTOMETRIC ANALYTICAL METHOD 1. Run the sample for spectrum 2. Obtain a monochromatic wavelength for the maximum absorption wavelength. 3. Calculate the concentration of your sample using Beer Lambert Equation: A = KCL

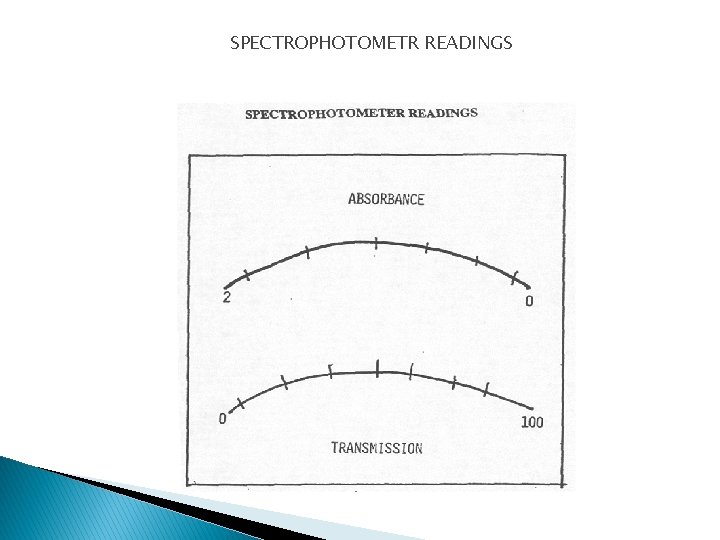
SPECTROPHOTOMETR READINGS

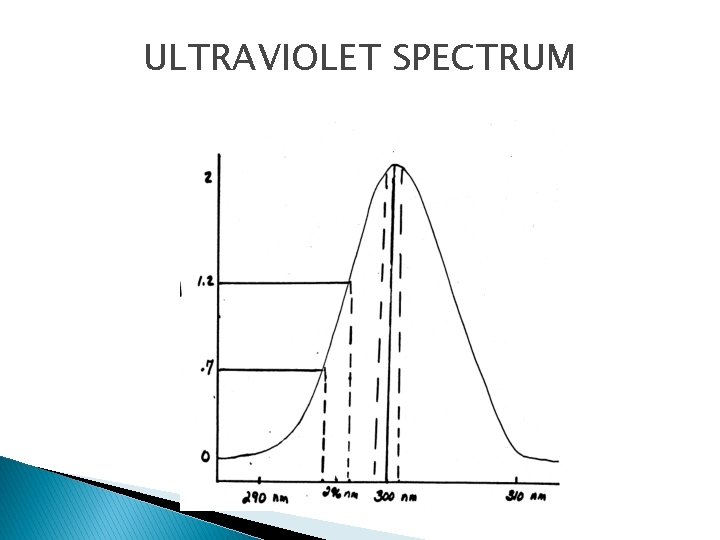
ULTRAVIOLET SPECTRUM

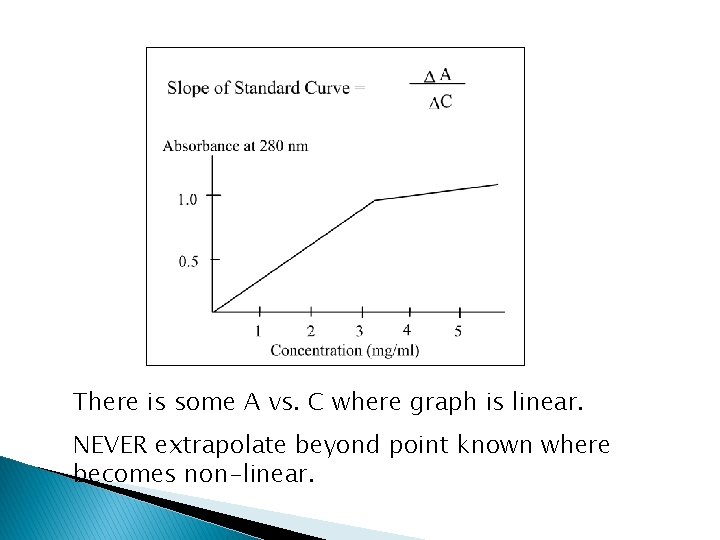
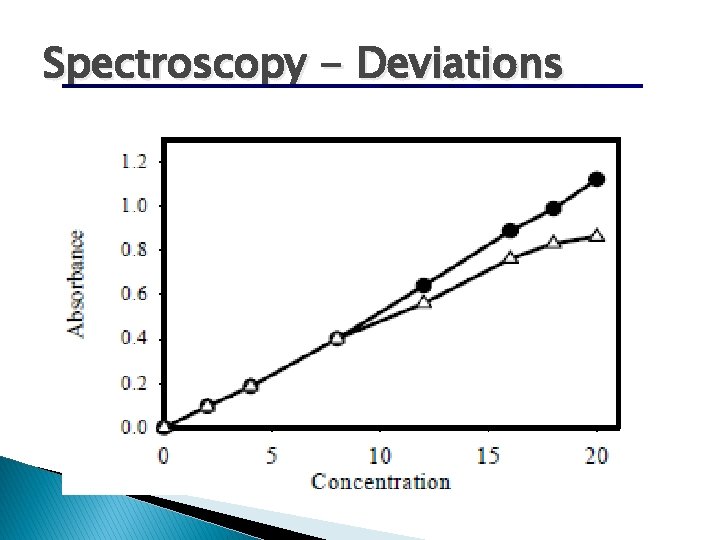
There is some A vs. C where graph is linear. NEVER extrapolate beyond point known where becomes non-linear.

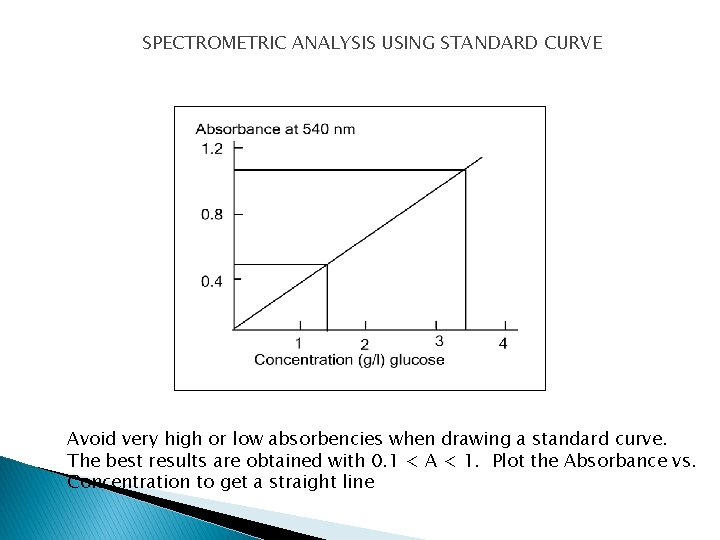
SPECTROMETRIC ANALYSIS USING STANDARD CURVE Avoid very high or low absorbencies when drawing a standard curve. The best results are obtained with 0. 1 < A < 1. Plot the Absorbance vs. Concentration to get a straight line

CELLS UV Spectrophotometer Quartz (crystalline silica) Visible Spectrophotometer Glass IR Spectrophotometer Na. Cl

LIGHT SOURCES UV Spectrophotometer 1. Hydrogen Gas Lamp 2. Mercury Lamp Visible Spectrophotometer 1. Tungsten Lamp IR Spectrophotometer 1. Carborundum (SIC)

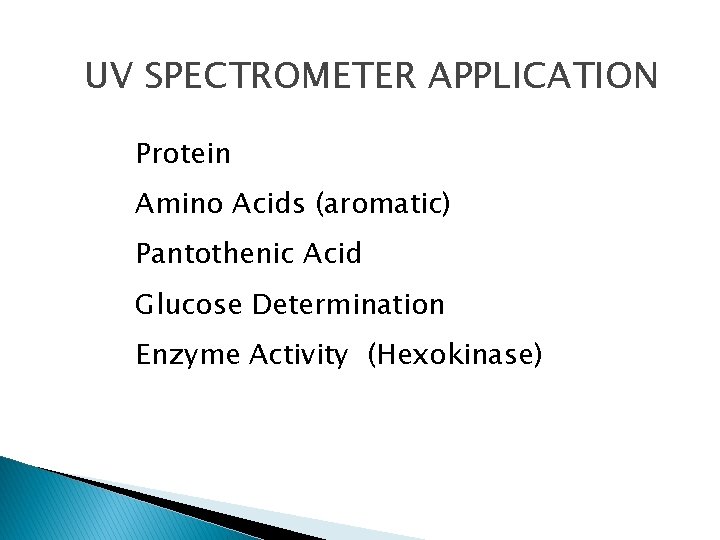
UV SPECTROMETER APPLICATION Protein Amino Acids (aromatic) Pantothenic Acid Glucose Determination Enzyme Activity (Hexokinase)

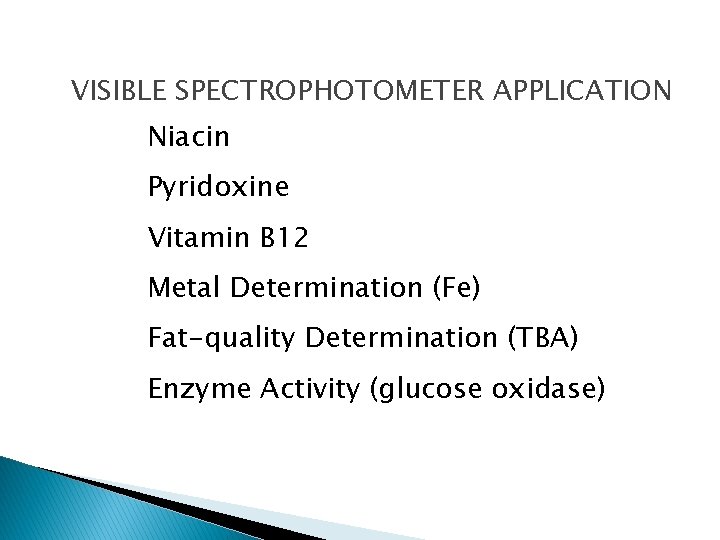
VISIBLE SPECTROPHOTOMETER APPLICATION Niacin Pyridoxine Vitamin B 12 Metal Determination (Fe) Fat-quality Determination (TBA) Enzyme Activity (glucose oxidase)

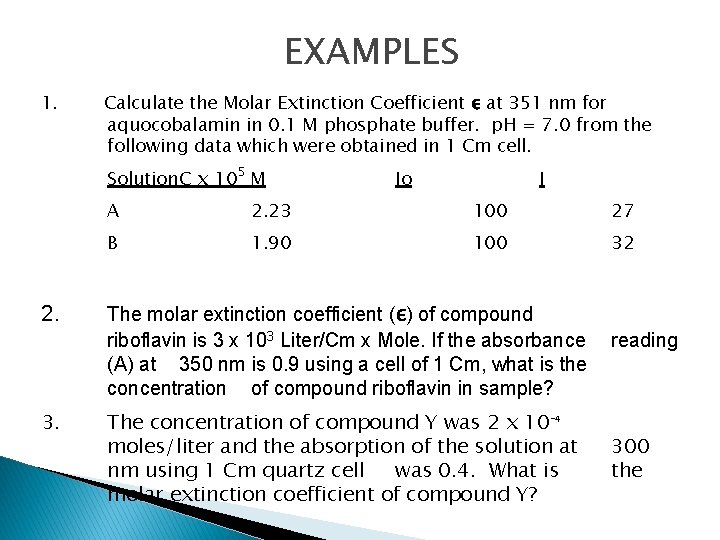
EXAMPLES 1. Calculate the Molar Extinction Coefficient ε at 351 nm for aquocobalamin in 0. 1 M phosphate buffer. p. H = 7. 0 from the following data which were obtained in 1 Cm cell. Solution. C x 105 M 2. 3. Io I A 2. 23 100 27 B 1. 90 100 32 The molar extinction coefficient (ε) of compound riboflavin is 3 x 103 Liter/Cm x Mole. If the absorbance reading (A) at 350 nm is 0. 9 using a cell of 1 Cm, what is the concentration of compound riboflavin in sample? The concentration of compound Y was 2 x 10 -4 moles/liter and the absorption of the solution at nm using 1 Cm quartz cell was 0. 4. What is molar extinction coefficient of compound Y? 300 the
 Absorbance Transmittance(%) ε(L/mole-cm) L(cm) 30 2000 1. 00](https://slidetodoc.com/presentation_image/2a48a965404c03efb838916f306fd8c5/image-22.jpg)
3. Complete the following table. [X](M) Absorbance Transmittance(%) ε(L/mole-cm) L(cm) 30 2000 1. 00 0. 5 2500 1. 00 2. 5 x 10 -3 0. 2 1. 00 4. 0 x 10 -5 5000 2. 0 x 10 -4 150 [X](M) = Concentration in Mole/L 4. The molar absorptivity of a pigment (molecular weight 300) is 30, 000 at 550 nm. What is the absorptivity in L/g-cm. ! 5. The iron complex of o-phenanthroline (Molecular weight 236) has molar absorptivity of 10, 000 at 525 nm. If the absorbance of 0. 01 is the lowest detectable signal, what concentration in part per million can be detected in a 1 -cm cell?

A = 0. 01 ε= 10000 L / mole x cm L = 1 cm A = ECL 0. 01= 10000 L/mole X Cm X C (Concentration) x 1 Cm C = mole / Liter C = X mole / Liter = X mole (236 g/mole) / Liter (1000 Cm 3) x PPM (10 -6 g/Cm 3) = X mole (236 g / mole) / Liter x 1 Liter / 1000 Cm 3 x ( PPM) 10 -6 g / Cm 3) =x PPM = 1 ug / Cm 3 1 ug = 10 -6 g

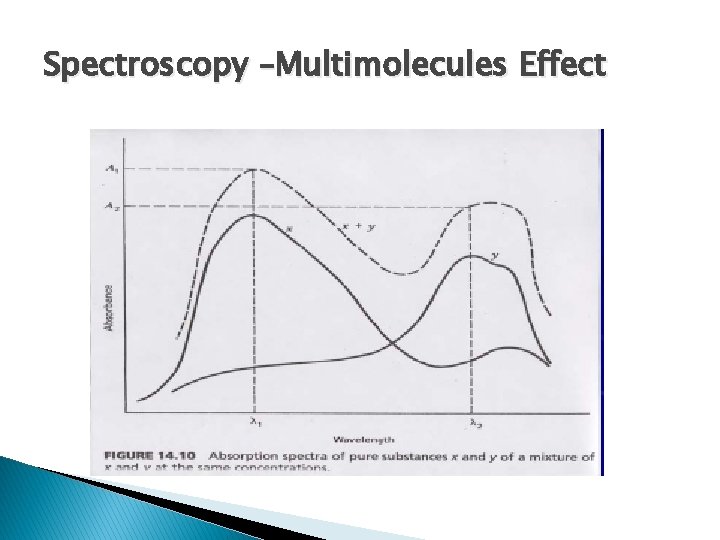
Spectroscopy –Multimolecules Effect

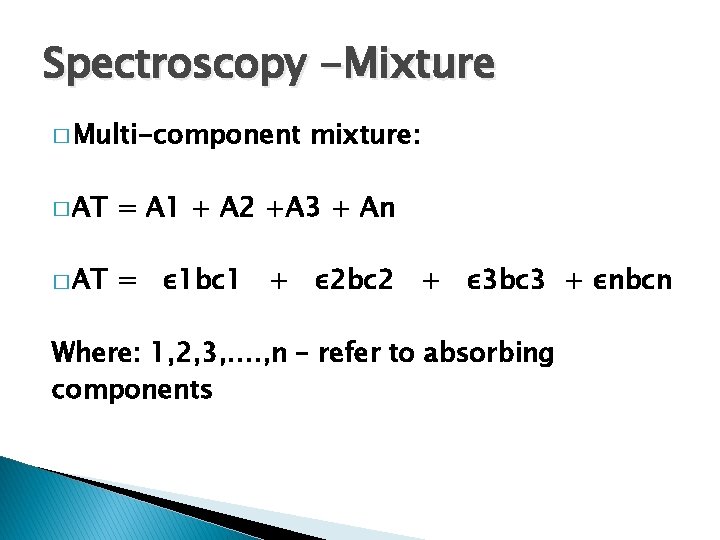
Spectroscopy -Mixture � Multi-component mixture: � AT = A 1 + A 2 +A 3 + An � AT = ε 1 bc 1 + ε 2 bc 2 + ε 3 bc 3 + εnbcn Where: 1, 2, 3, …. , n – refer to absorbing components

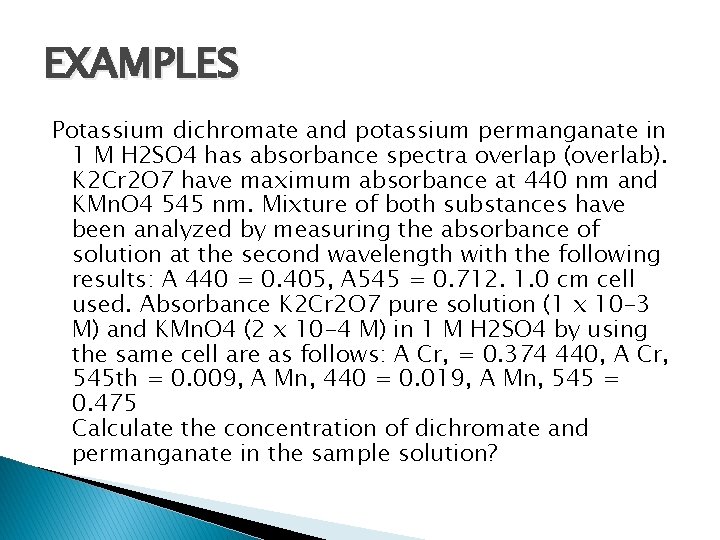
EXAMPLES Potassium dichromate and potassium permanganate in 1 M H 2 SO 4 has absorbance spectra overlap (overlab). K 2 Cr 2 O 7 have maximum absorbance at 440 nm and KMn. O 4 545 nm. Mixture of both substances have been analyzed by measuring the absorbance of solution at the second wavelength with the following results: A 440 = 0. 405, A 545 = 0. 712. 1. 0 cm cell used. Absorbance K 2 Cr 2 O 7 pure solution (1 x 10 -3 M) and KMn. O 4 (2 x 10 -4 M) in 1 M H 2 SO 4 by using the same cell are as follows: A Cr, = 0. 374 440, A Cr, 545 th = 0. 009, A Mn, 440 = 0. 019, A Mn, 545 = 0. 475 Calculate the concentration of dichromate and permanganate in the sample solution?

Spectroscopy – Beer’s Law Limitations Deviations from the direct �proportionality (b=const) �Instrumental Deviations �Chemical Deviations

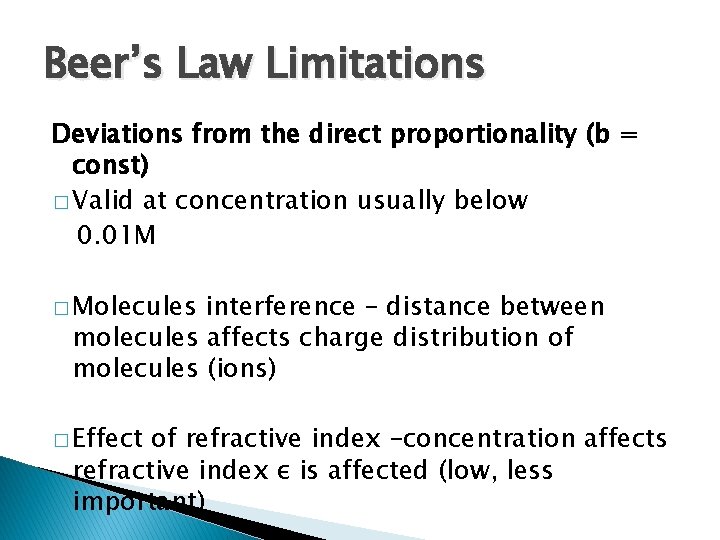
Beer’s Law Limitations Deviations from the direct proportionality (b = const) � Valid at concentration usually below 0. 01 M � Molecules interference – distance between molecules affects charge distribution of molecules (ions) � Effect of refractive index –concentration affects refractive index ε is affected (low, less important)

Spectroscopy - Deviations

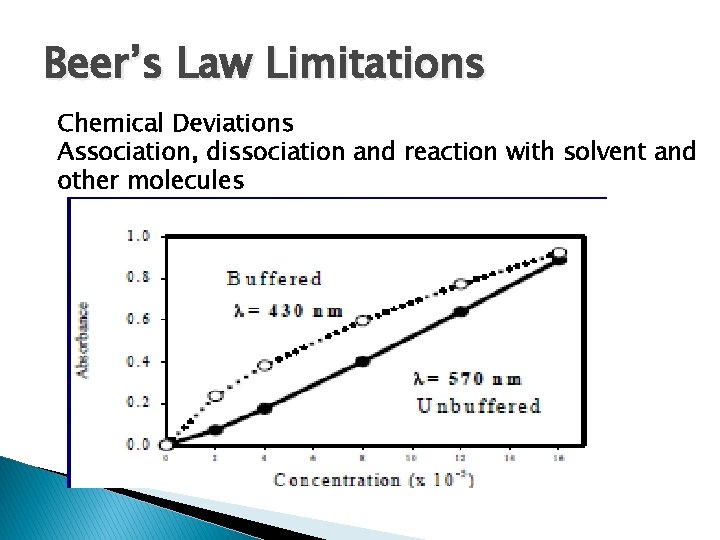
Beer’s Law Limitations Chemical Deviations Association, dissociation and reaction with solvent and other molecules

Beer’s Law Limitations Instrumental Deviations � monochromatic radiation – quality of monochromator and control of bandwidth and slit � Instrumental noise – accuracy of measurement of transmittance – quality of detector