Chapter 14 The Interstellar Medium ISM All of





















- Slides: 32

Chapter 14 The Interstellar Medium

ISM • All of the material other than stars, planets, and degenerate objects • Composed of gas and dust • ~1% of the mass of the galaxy • Site of star formation

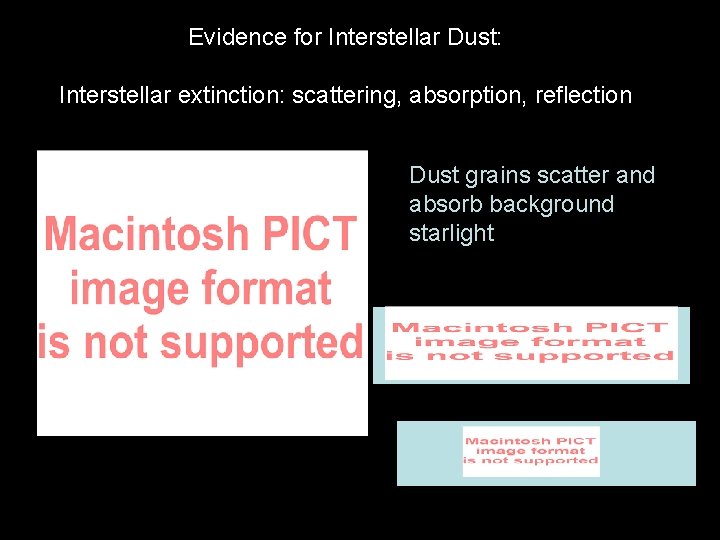
Evidence for Interstellar Dust: Interstellar extinction: scattering, absorption, reflection Dust grains scatter and absorb background starlight

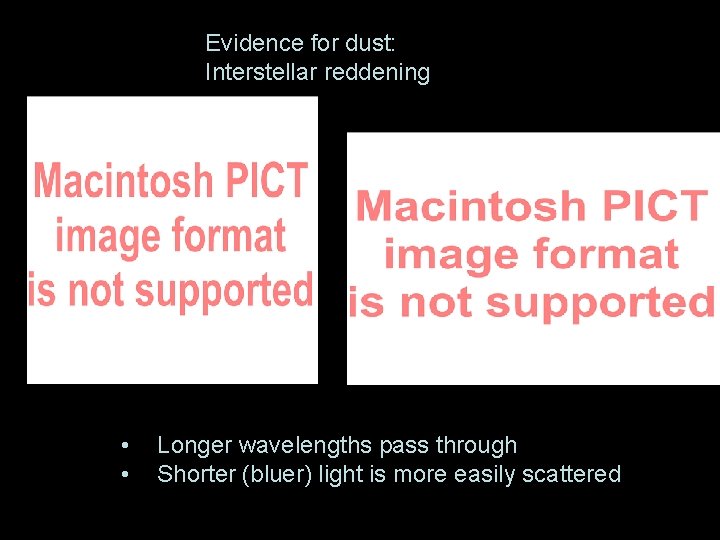
Evidence for dust: Interstellar reddening • • Longer wavelengths pass through Shorter (bluer) light is more easily scattered

Interstellar extinction curve o 2200 A bump

The densest ISM: Molecular clouds, birthplace of stars

Gas and dust collect into clouds: Dust radiates in thermal infrared

Phases of the ISM Compare the pressures (P=nk. T) of each of these phases!

Comparing the pressures of different phases of ISM

The Milky Way at 100 microns Warm dust traces out the ISM

Observing cold neutral hydrogen, HI (“H-one”) Interaction between electron spin and nuclear spin

21 -cm emission from the entire Milky Way

Spectroscopy of molecular clouds • • First detected in 1937 !!! 146 compounds have been identified in the ISM as of June 2006 Most are organic (contain C and at least one atom other than O) List does not include deuterated species or ions Good reference: http: //www. cv. nrao. edu/~awootten/allmols. html More than half originally detected in Sgr B 2 (massive star forming region near galactic center) My personal faves: glycine, ethanol, acetic acid !!

Compounds are identified by their rotational spectrum Sgr B 2

The rich molecular spectrum of Sgr B 2

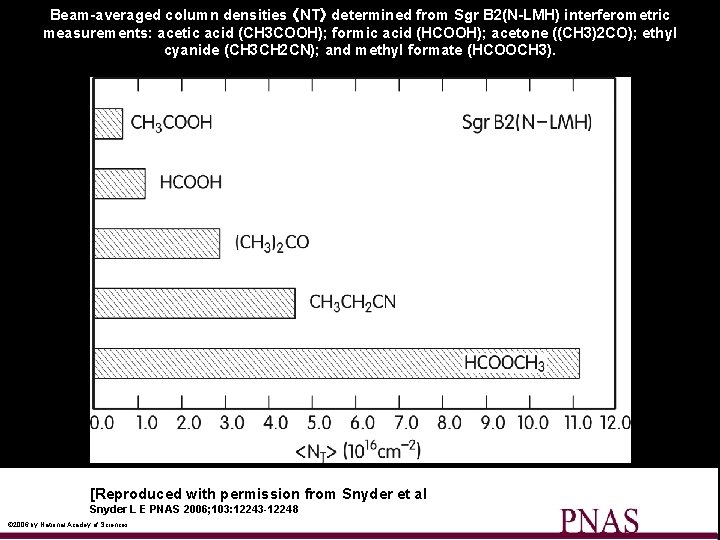
Beam-averaged column densities 〈NT〉 determined from Sgr B 2(N-LMH) interferometric measurements: acetic acid (CH 3 COOH); formic acid (HCOOH); acetone ((CH 3)2 CO); ethyl cyanide (CH 3 CH 2 CN); and methyl formate (HCOOCH 3). [Reproduced with permission from Snyder et al Snyder L E PNAS 2006; 103: 12243 -12248 © 2006 by National Acadey of Sciences

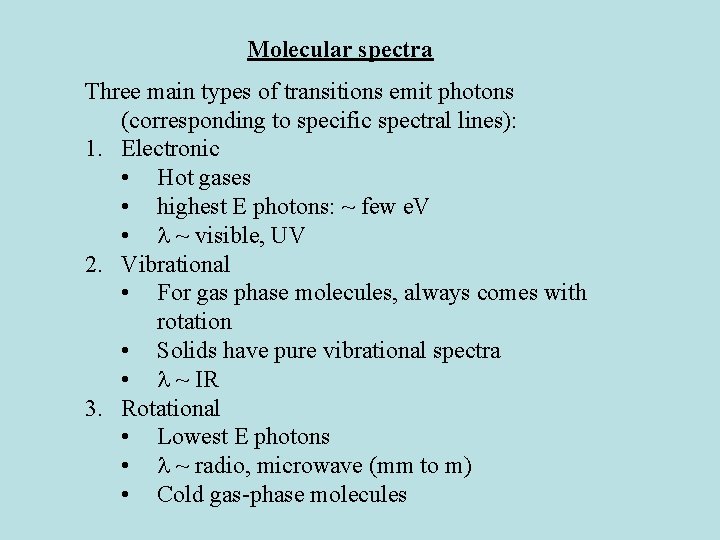
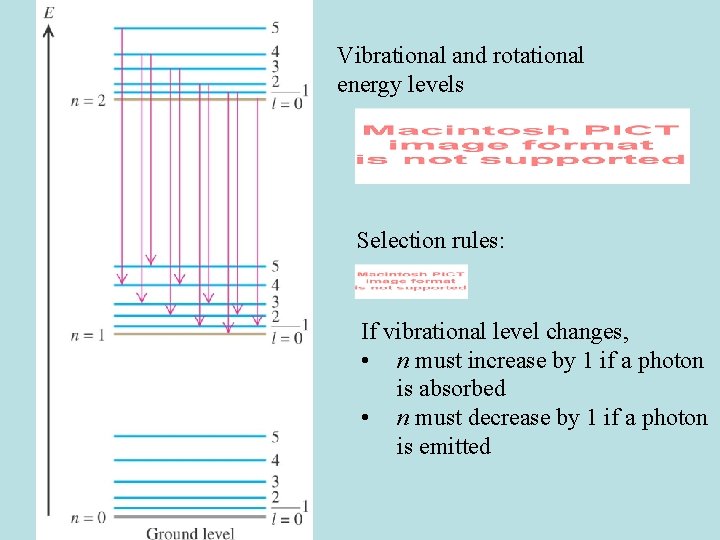
Molecular spectra Three main types of transitions emit photons (corresponding to specific spectral lines): 1. Electronic • Hot gases • highest E photons: ~ few e. V • ~ visible, UV 2. Vibrational • For gas phase molecules, always comes with rotation • Solids have pure vibrational spectra • ~ IR 3. Rotational • Lowest E photons • ~ radio, microwave (mm to m) • Cold gas-phase molecules

Rotational spectra

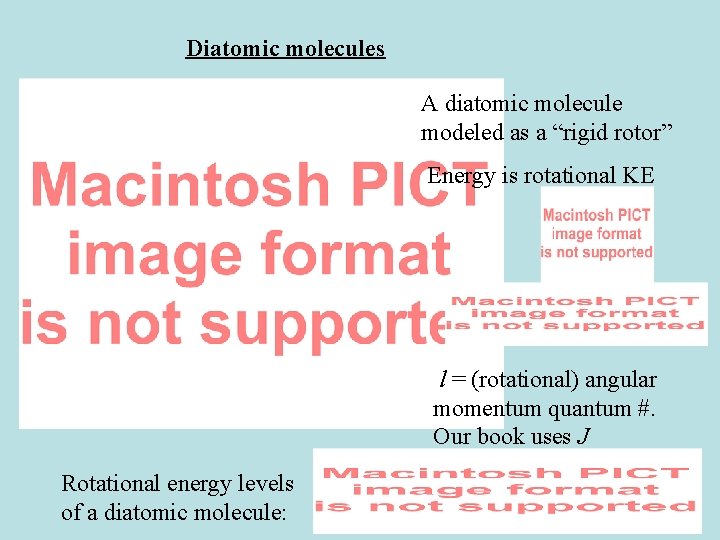
Diatomic molecules A diatomic molecule modeled as a “rigid rotor” Energy is rotational KE l = (rotational) angular momentum quantum #. Our book uses J Rotational energy levels of a diatomic molecule:

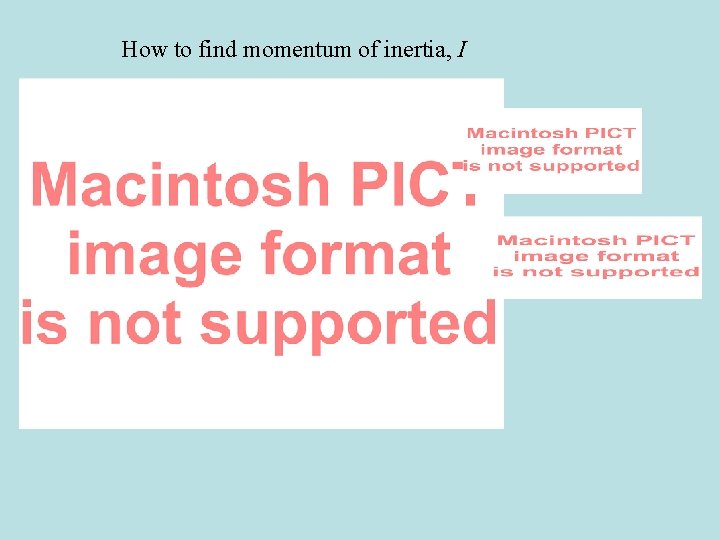
How to find momentum of inertia, I

Rotational energy levels for a diatomic molecule **Our book uses J instead of l


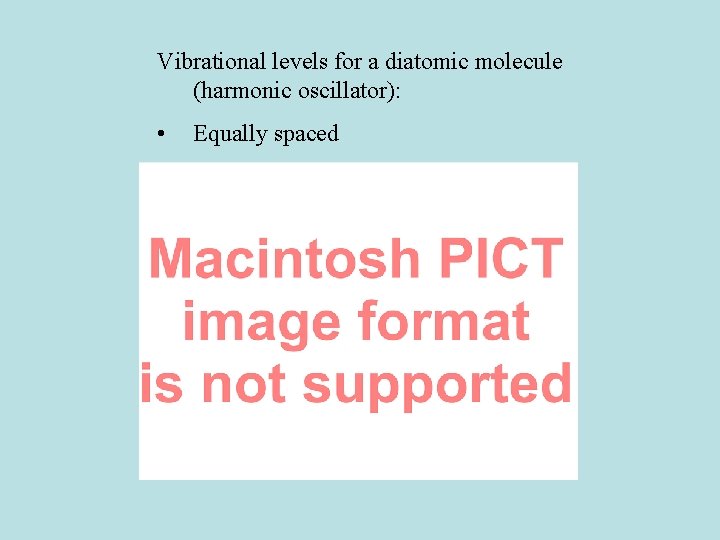
Vibrational energy levels n = vibrational quantum number

Vibrational levels for a diatomic molecule (harmonic oscillator): • Equally spaced




Formation of interstellar molecules • • Hydrogenated (H 2 O, CH 4, NH 3) CO, CO 2, N 2, etc

Vibrational and rotational energy levels Selection rules: If vibrational level changes, • n must increase by 1 if a photon is absorbed • n must decrease by 1 if a photon is emitted

Rotation-vibration spectrum of HCl

Rotation-vibration spectrum of HCl

Radio Spectrum of a molecular cloud