Chapter 11 Protein Proteins are mainly composed of







- Slides: 15

Chapter 11 Protein • Proteins are mainly composed of the elements carbon, hydrogen, oxygen, and nitrogen • Proteins are made up of subunits called organic acids which contain carboxyl groups (-COOH) • Organic acids in proteins are called amino acids

Amino Acids • Amino acids have three parts to their structure; a side chain of carbon and hydrogen atoms, a carboxyl group, and an amine group. • An amine group contains one nitrogen and two hydrogen atoms, (-NH 2) • When two amino acids join together the bond is called a peptide bond

Amino Acids • There are 20 amino acids that are necessary for the body to remain healthy, grow, and maintain body functions • Eight of these amino acids cannot be produced in the body and are classified as essential amino acids • Essential amino acids- amino acids that must be supplied by foods in the diet

Complete vs. Incomplete Proteins • Complete proteins- foods that contain all 8 essential amino acids • Most come from animal sources and are high quality proteins, ex. eggs, milk, fish, poultry, meats • Incomplete proteins- foods that are short of one or more of the 8 essential amino acids needed for human growth • Most come from grains, nuts, vegetables, and legumes

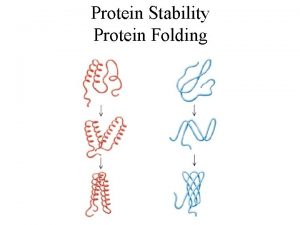
Protein Structures • There are infinite numbers of protein structures but they can be classified in three ways. • 1. Primary structure • 2. Secondary structure • 3. Tertiary structure

Primary Structure • Primary structure of a protein molecule is the order the amino acids occur in the chain • A result of the chain of peptide bonds formed in making the protein molecule

Secondary Structure • Secondary structure of a protein molecule refers to the shape of sections of the amino acid chain • The three patterns of secondary structures are the helix, random coil, and the pleated sheet

Tertiary Structure • Tertiary structure of a protein molecule refers to the three-dimensional structure of an entire amino acid chain • The two main tertiary structures are globular (balled up) and fibrous (strands) • Globular proteins- hemoglobin, lipoprotein, casein, and albumin • Fibrous proteins- helix shaped strands, ex. collagen, elastin, keratin, myosin are all found in muscle fibers, ligaments, tendons, hair and fingernails

Hydrophobic Interactions • A common interaction between protein molecules is a hydrophobic, or water repelling, interaction that occurs between nonpolar side chains with carbon rings • Casein, found in milk, is an example of a hydrophobic protein and is vital to the formation of curds in cheese making

Oxidation and Reduction • The reversible process of adding and removing oxygen to a compound is called oxidation and reduction • Oxidation adds oxygen to a compound • Reduction removes oxygen from a compound

Denaturation of Proteins • Denaturation- Any change of the shape of a protein molecule without breaking peptide bonds and usually results in a loosening or unfolding of the protein molecule • Denaturation is occasionally reversible but it is not when disulfide cross-links are broken

Causes of Denaturation • • Hot and cold temperatures Mechanical actions Sound waves Pressure Irradiation p. H changes Mineral salts

Heat and Denaturation • The rate of increase for protein denaturation is 600 times for every 10ºC increase in temperature

Coagulation • Coagulation- a type of permanent denaturation when a liquid or semiliquid protein forms solid or semisoft clots. • Example- milk curdling to form cheese

 Insidan region jh
Insidan region jh Composed mainly of minerals with blocky crystal shapes
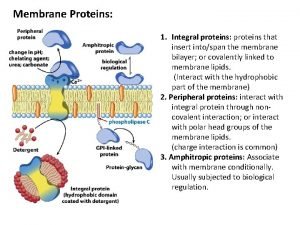
Composed mainly of minerals with blocky crystal shapes Carrier vs channel proteins
Carrier vs channel proteins Protein-protein docking
Protein-protein docking Where is hinduism located today
Where is hinduism located today The earliest bridges consisted mainly of
The earliest bridges consisted mainly of Chapter 38 principles of baking
Chapter 38 principles of baking Various theories of breakdown in liquid dielectrics
Various theories of breakdown in liquid dielectrics What reaction does akira most fear from chie
What reaction does akira most fear from chie Oedipus rex mcq
Oedipus rex mcq Oedipus rex practice multiple choice questions answers
Oedipus rex practice multiple choice questions answers Adulteration of dal with kesari dal mainly causes
Adulteration of dal with kesari dal mainly causes Mud box in boiler
Mud box in boiler Project procurement management mainly involves
Project procurement management mainly involves Refraction of light
Refraction of light Diagrams tables and graphs are used by scientists mainly to
Diagrams tables and graphs are used by scientists mainly to