Changes of State Changes of State Is the







- Slides: 10

Changes of State

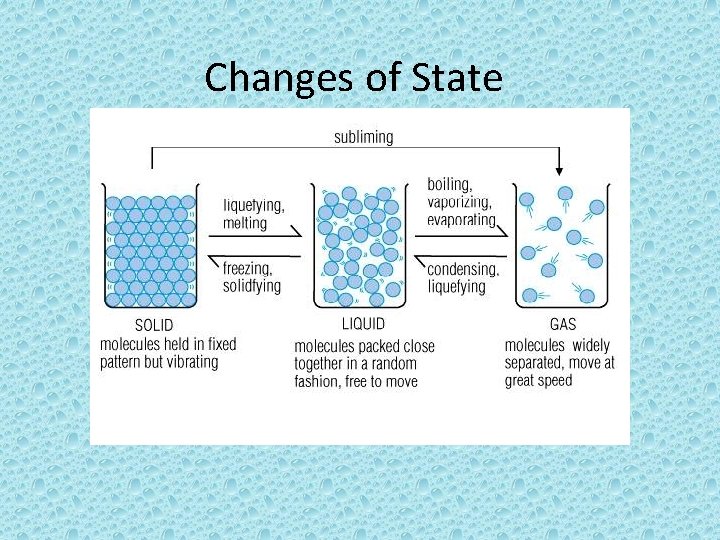
Changes of State • Is the change of a substance from one physical form to the other • All changes of states are physical changes, this means that the identity of a substance does not change – Ex. Ice turns to water through melting. It is still made of the same thing.

Energy • Particles of a substance have different amounts of energy when the substance is in different states. – Ex. Particles in Liquid water have more energy than particles in ice • When a change of state happens, the energy of the particles of the substance changes too. • So to change state you must add or remove energy

Melting • Is the change of state from a solid to a liquid. • You must add energy to a solid to increase the temperature. As it heats up the particles of the solid speed up. • The temperature at which a substance changes from a solid to a liquid is the Melting point. • Different substances have different melting points

Melting • For a solid to melt, particles must overcome their attraction to each other. • Melting is endothermic change because energy is gained by the substance as it changes.

Freezing • Change of state from a liquid to a solid • The temperature at which a liquid changes into a solid is the freezing point • Freezing and melting happen at the same temperature • To turn from a liquid to a solid the particles must overcome the motion particles

Freezing • Freezing is an exothermic change because energy is removed from, or taken out of the substance as it changes

Vaporization • Change of state from a liquid to a gas • Two types of Vaporization • Takes place throughout a liquid is called boiling • The temperature at which a liquid boils is called the boiling point • Evaporation occurs at the surface of a liquid below boiling point.

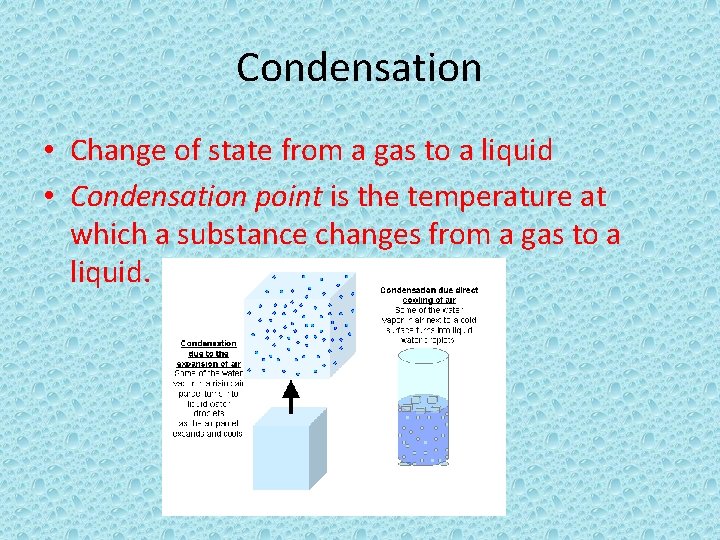
Condensation • Change of state from a gas to a liquid • Condensation point is the temperature at which a substance changes from a gas to a liquid.

Sublimation • Change of state from a solid directly to a gas. • Ex. Dry ice is colder than ice. Unlike ice, dry ice does not melt into a puddle of liquid.