CH 339 K SOMETHING COOL Wooly Mammoth Mammuthus




- Slides: 37

CH 339 K SOMETHING COOL

Wooly Mammoth – Mammuthus primigenius • • Disappeared about 10, 000 BC Frozen remains found periodically Wooly mammoth hemoglobin reconstructed Campbell, K. L. et al. (2010) Nature Genetics Advance Online Publication

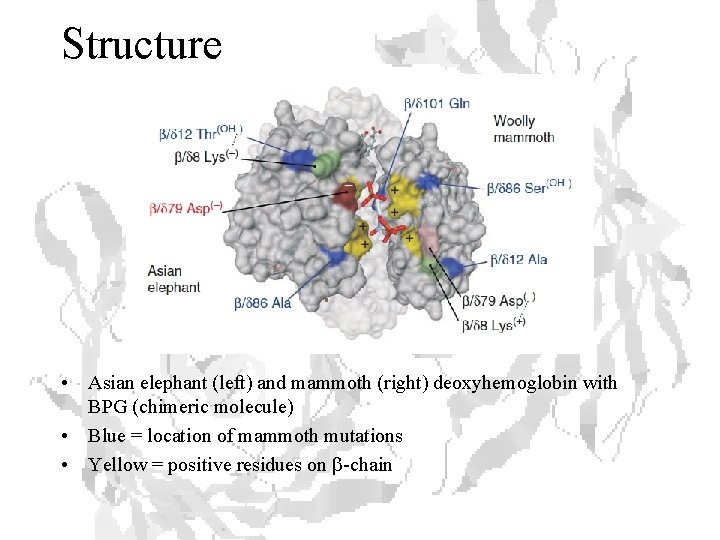
Structure • Asian elephant (left) and mammoth (right) deoxyhemoglobin with BPG (chimeric molecule) • Blue = location of mammoth mutations • Yellow = positive residues on b-chain

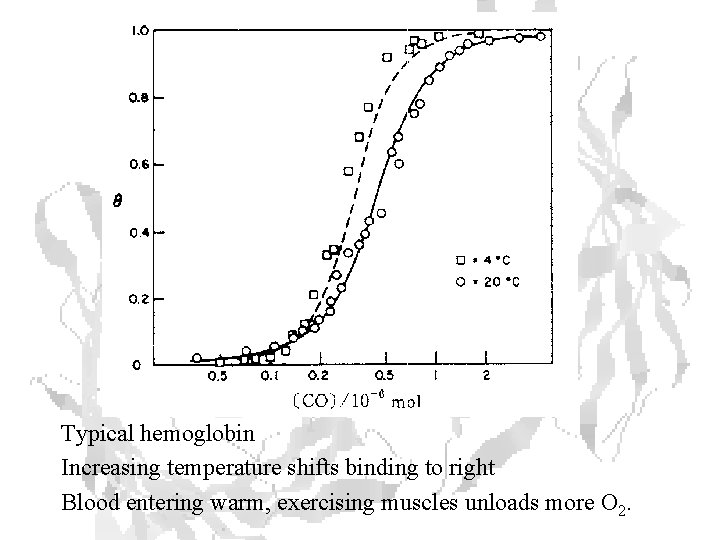
Typical hemoglobin Increasing temperature shifts binding to right Blood entering warm, exercising muscles unloads more O 2.

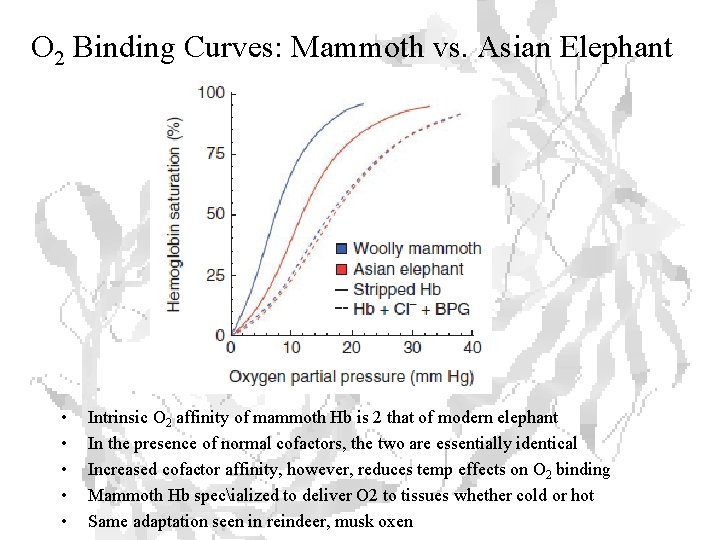
O 2 Binding Curves: Mammoth vs. Asian Elephant • • • Intrinsic O 2 affinity of mammoth Hb is 2 that of modern elephant In the presence of normal cofactors, the two are essentially identical Increased cofactor affinity, however, reduces temp effects on O 2 binding Mammoth Hb specialized to deliver O 2 to tissues whether cold or hot Same adaptation seen in reindeer, musk oxen

CH 339 K PHOTOSYNTHESIS

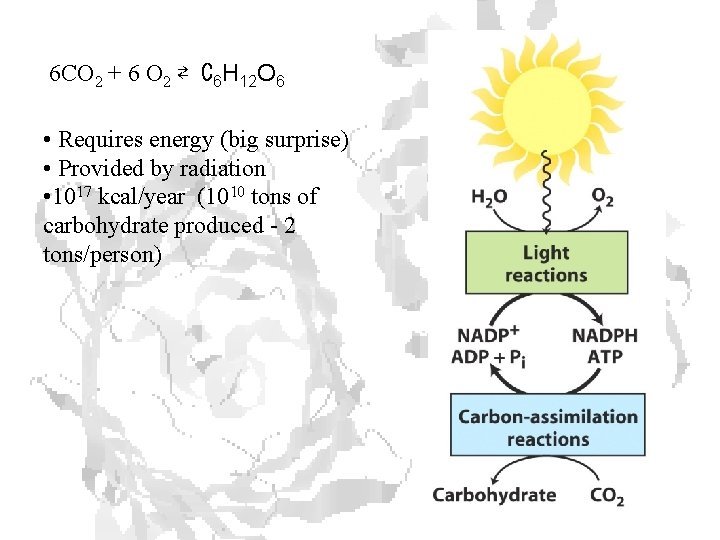
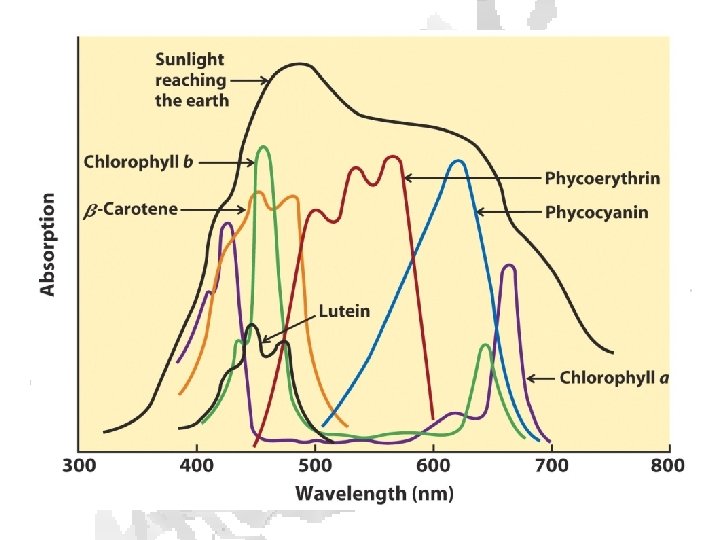
6 CO 2 + 6 O 2 ⇄ C 6 H 12 O 6 • Requires energy (big surprise) • Provided by radiation • 1017 kcal/year (1010 tons of carbohydrate produced - 2 tons/person)



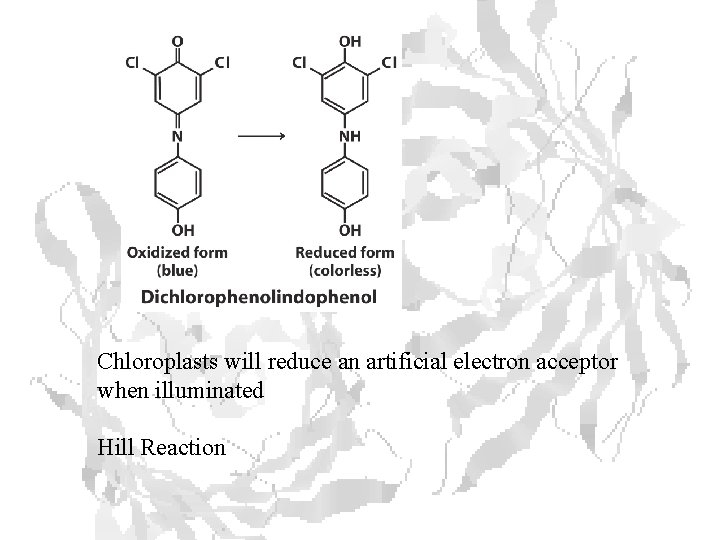
Chloroplasts will reduce an artificial electron acceptor when illuminated Hill Reaction

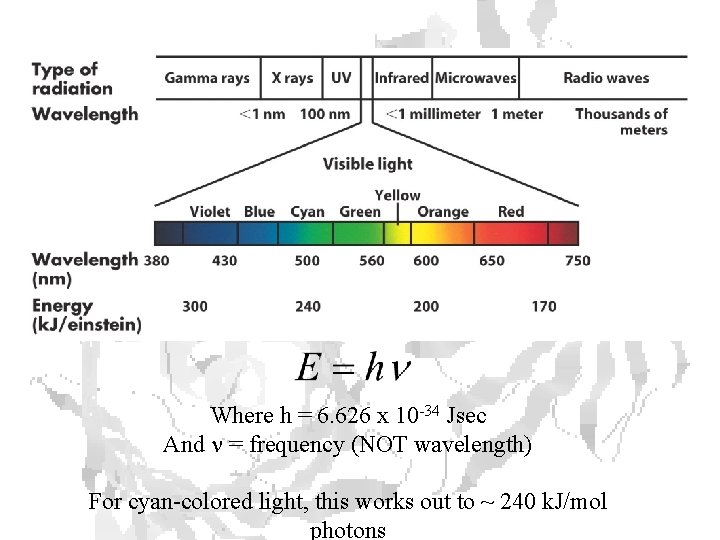
Where h = 6. 626 x 10 -34 Jsec And n = frequency (NOT wavelength) For cyan-colored light, this works out to ~ 240 k. J/mol photons

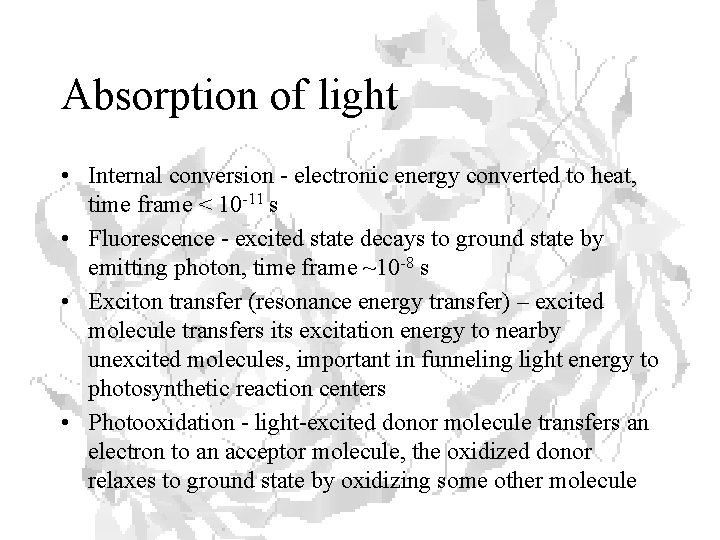
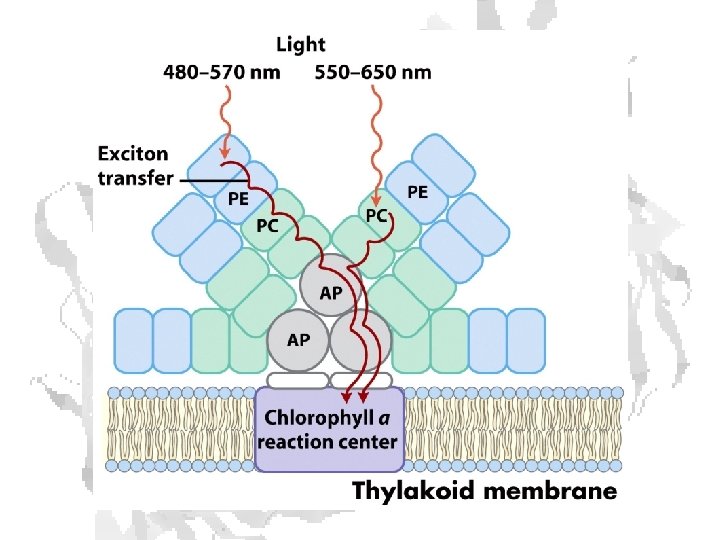
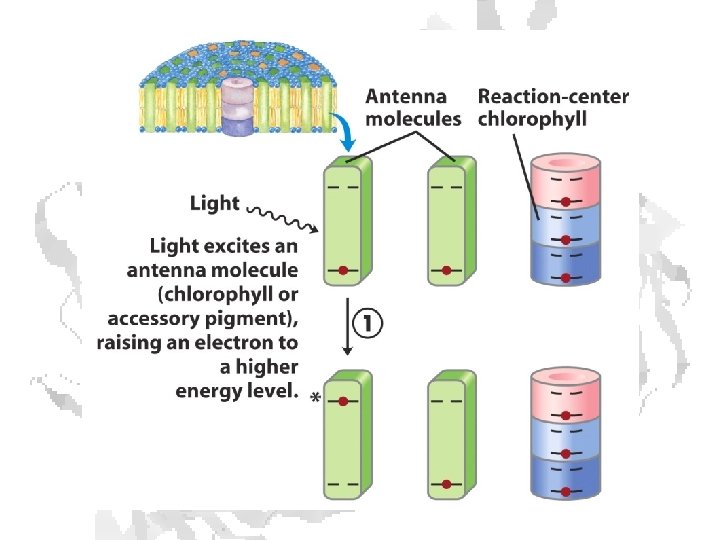
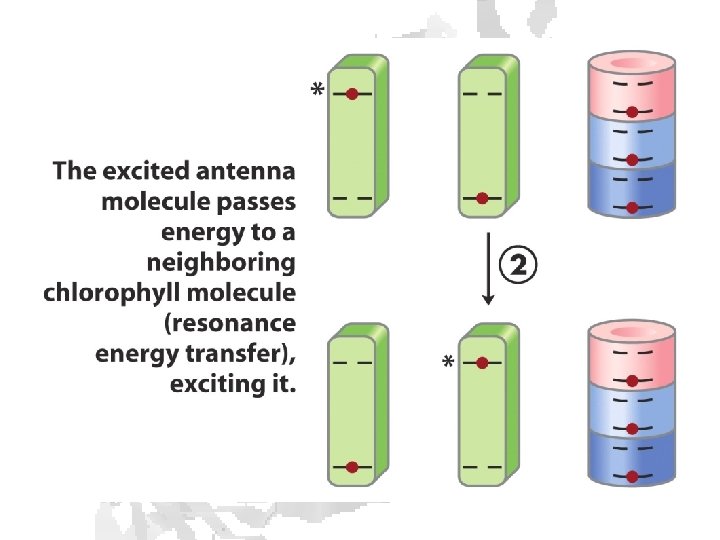
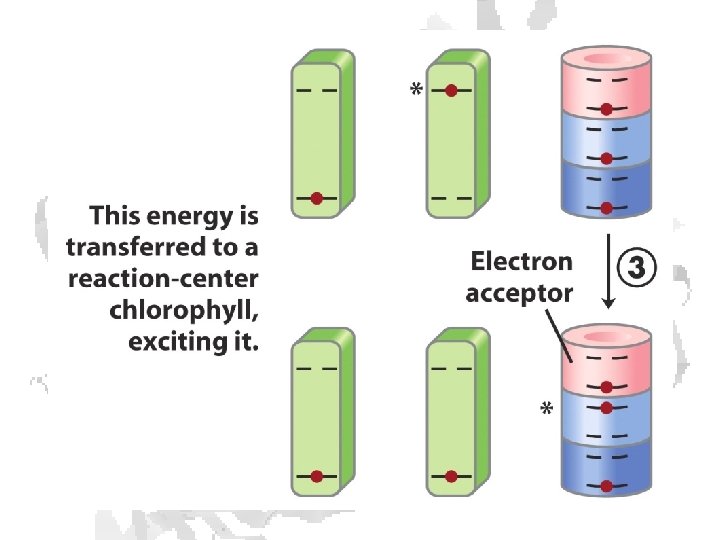
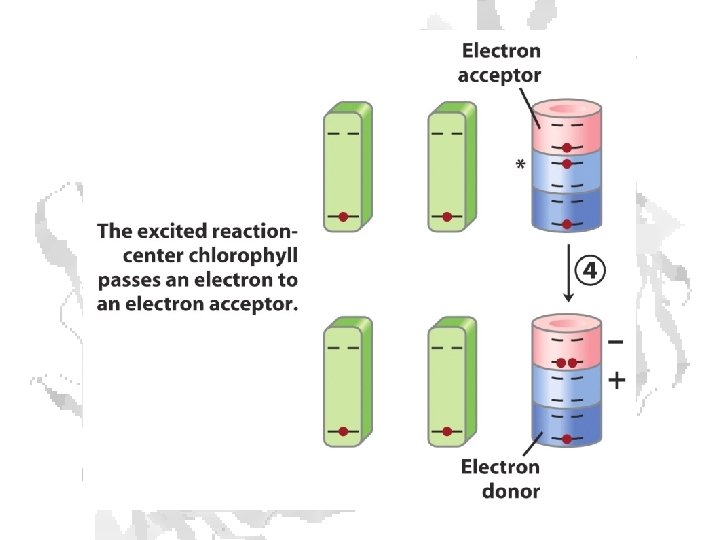
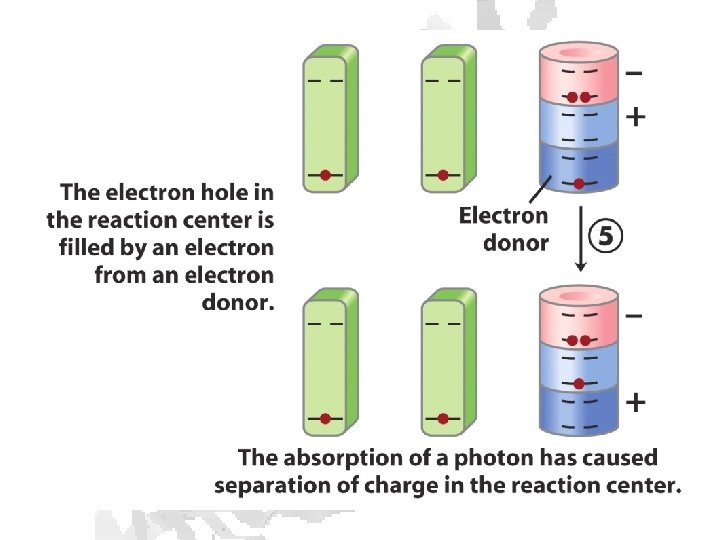
Absorption of light • Internal conversion - electronic energy converted to heat, time frame < 10 -11 s • Fluorescence - excited state decays to ground state by emitting photon, time frame ~10 -8 s • Exciton transfer (resonance energy transfer) – excited molecule transfers its excitation energy to nearby unexcited molecules, important in funneling light energy to photosynthetic reaction centers • Photooxidation - light-excited donor molecule transfers an electron to an acceptor molecule, the oxidized donor relaxes to ground state by oxidizing some other molecule






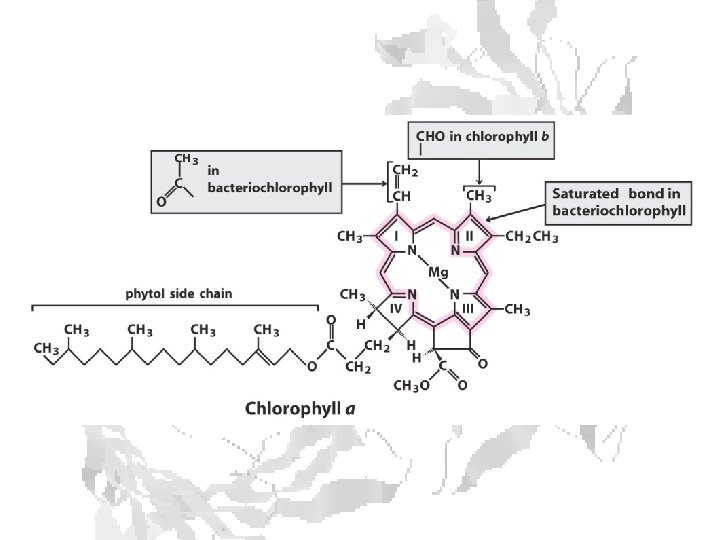
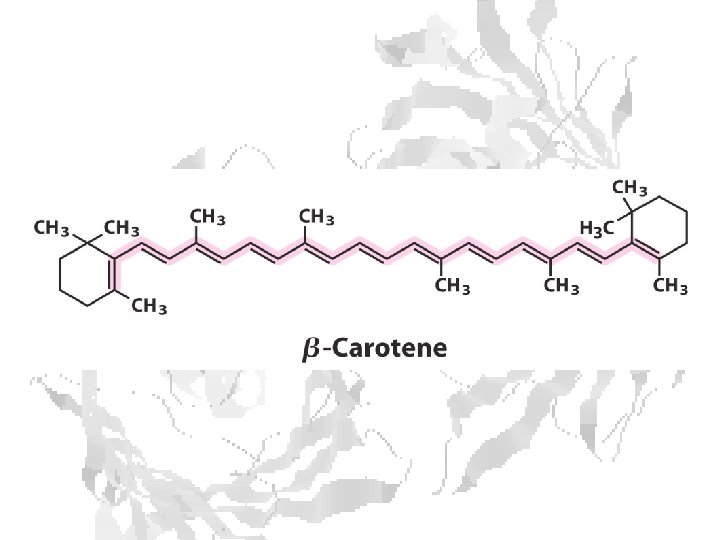
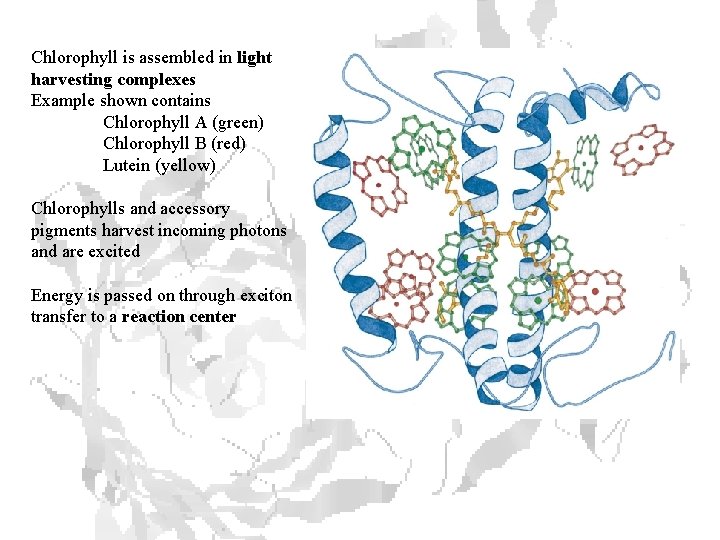
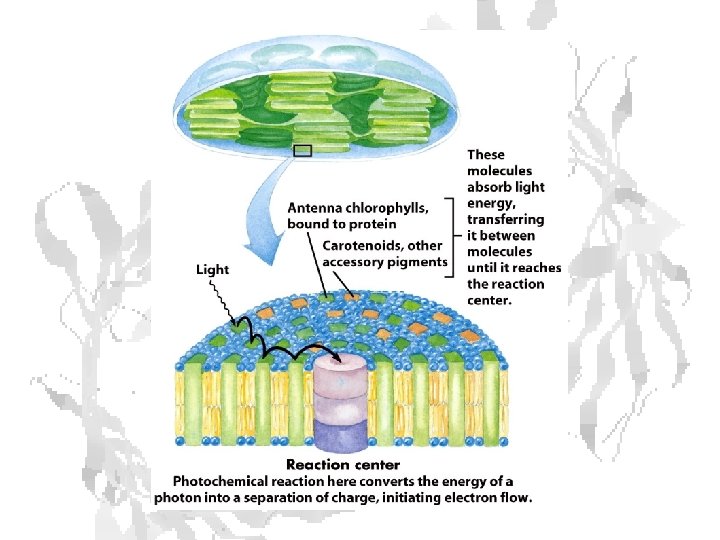
Chlorophyll is assembled in light harvesting complexes Example shown contains Chlorophyll A (green) Chlorophyll B (red) Lutein (yellow) Chlorophylls and accessory pigments harvest incoming photons and are excited Energy is passed on through exciton transfer to a reaction center








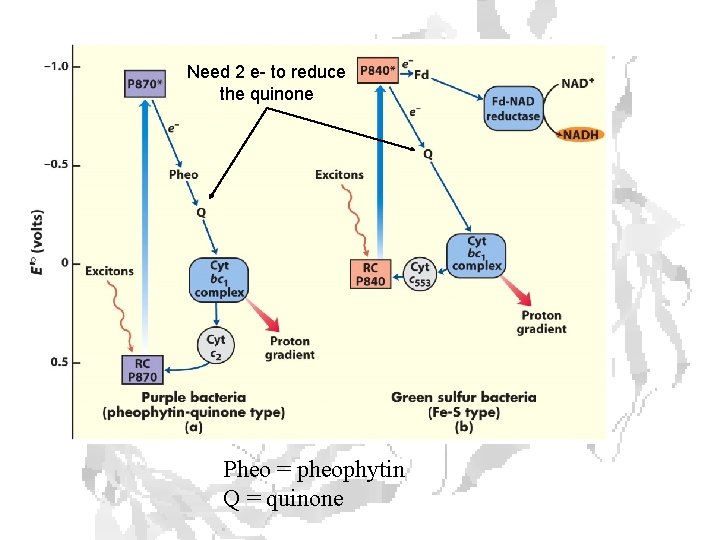
Need 2 e- to reduce the quinone Pheo = pheophytin Q = quinone

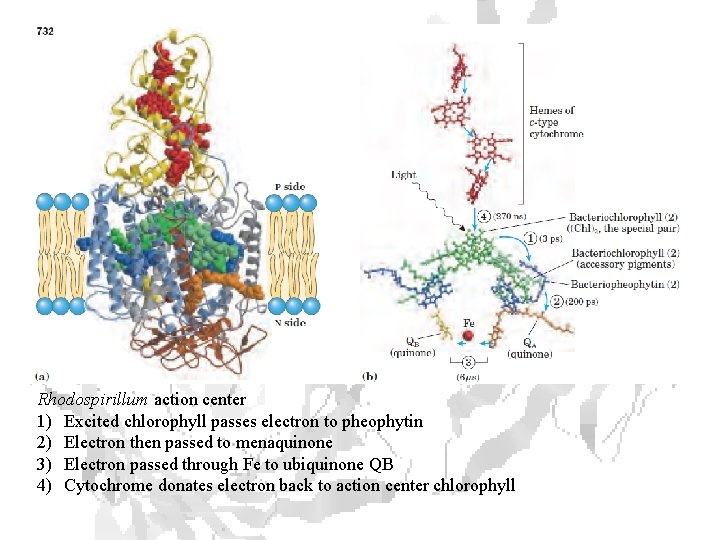
Rhodospirillum action center 1) Excited chlorophyll passes electron to pheophytin 2) Electron then passed to menaquinone 3) Electron passed through Fe to ubiquinone QB 4) Cytochrome donates electron back to action center chlorophyll

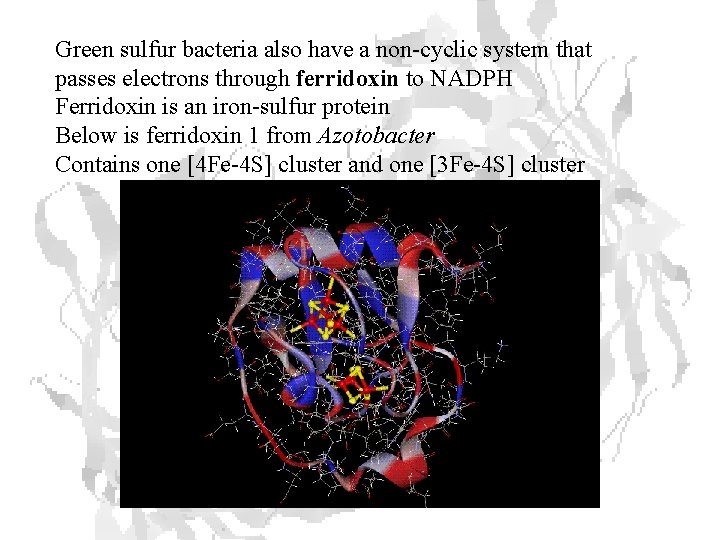
Green sulfur bacteria also have a non-cyclic system that passes electrons through ferridoxin to NADPH Ferridoxin is an iron-sulfur protein Below is ferridoxin 1 from Azotobacter Contains one [4 Fe-4 S] cluster and one [3 Fe-4 S] cluster

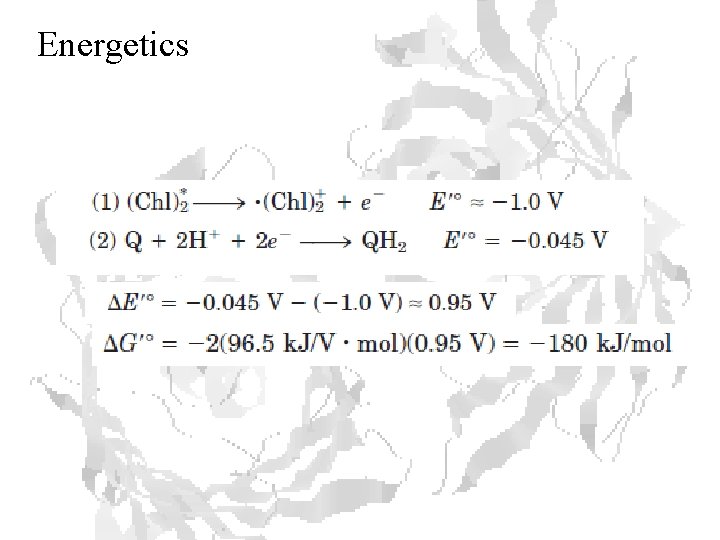
Energetics

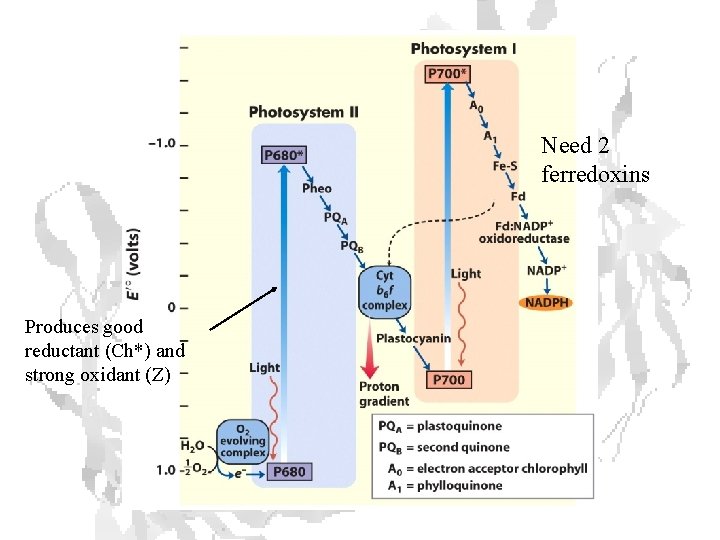
Need 2 ferredoxins Produces good reductant (Ch*) and strong oxidant (Z)


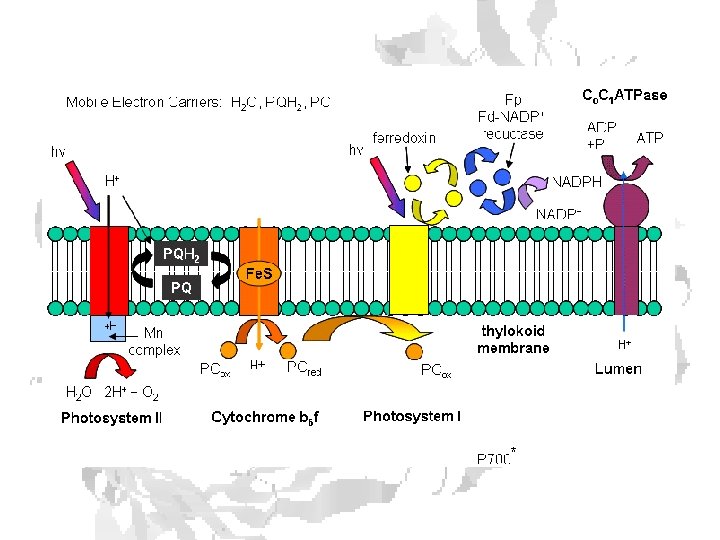
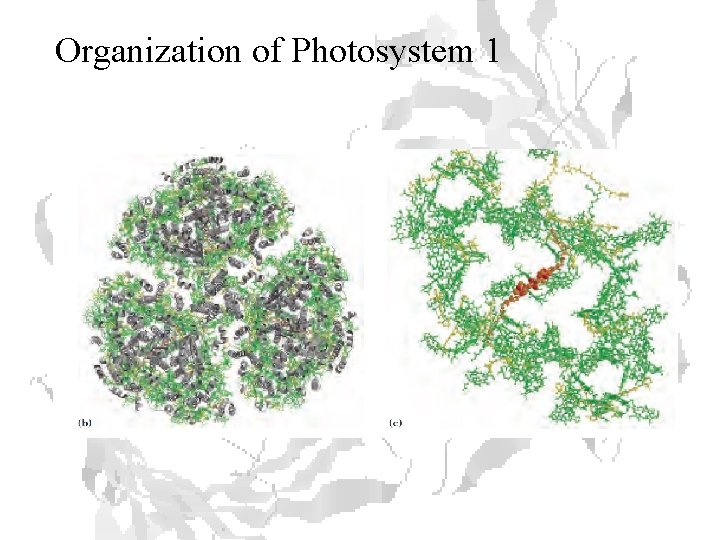
Organization of Photosystem 1

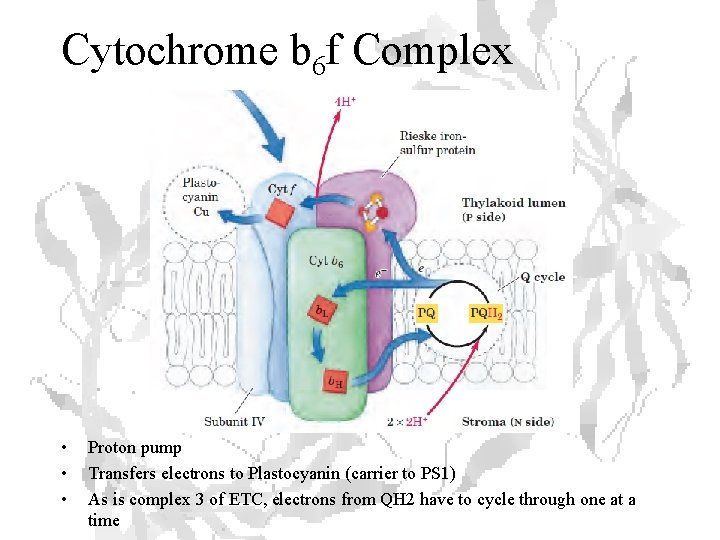
Cytochrome b 6 f Complex • • • Proton pump Transfers electrons to Plastocyanin (carrier to PS 1) As is complex 3 of ETC, electrons from QH 2 have to cycle through one at a time

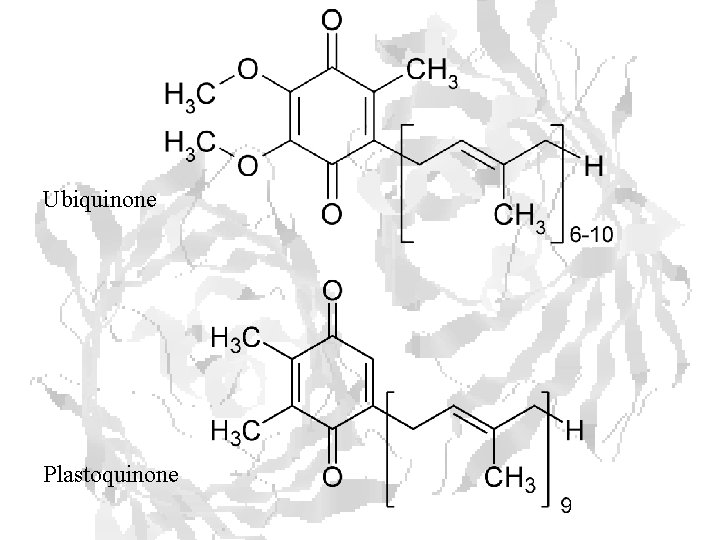
Ubiquinone Plastoquinone

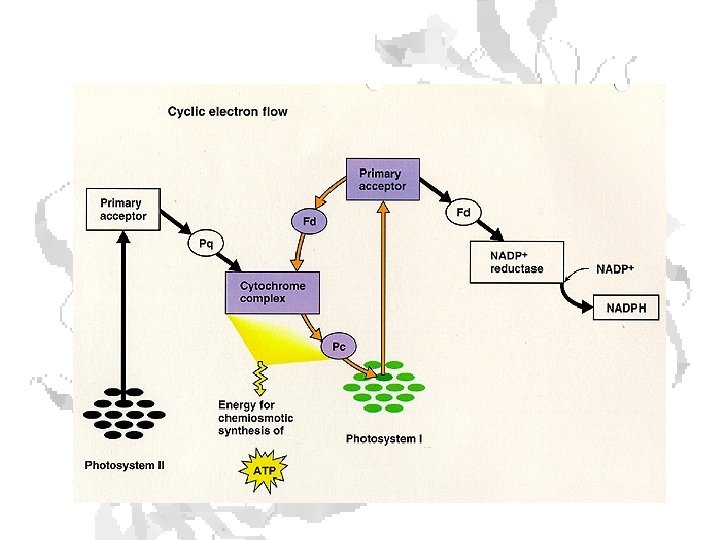
Cyclic Photophosphorylation To fix 1 CO 2 requires: 2 NADPH molecules 3 ATP molecules • Each molecule of oxygen released by the light reactions supplies the 4 electrons needed to make 2 NADPH molecules. • 4 electrons passingthrough cytochrome b 6/f complex provides enough energy to pump 12 protons into the interior of the thylakoid. • To make 3 molecules of ATP, the ATPase in chloroplasts needs about 14 protons (H+) Deficit is made up by cyclic photophosphorylation. • Electrons expelled by the energy of light absorbed by photosystem I pass, as normal, to ferredoxin (Fd). • Then pass to plastoquinone (PQ) and on back into the cytochrome b 6/f complex. • Here each electron liberates pumps 2 protons (H+) into the interior of the thylakoid — enough to make up the deficit left by noncyclic photophosphorylation.


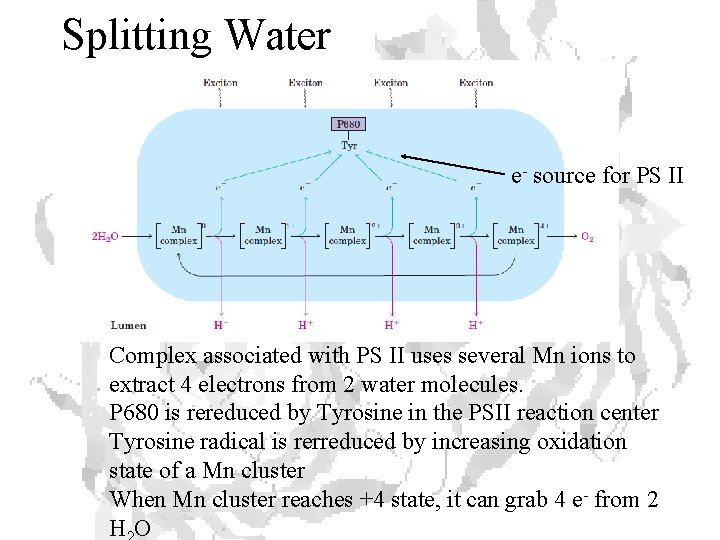
Splitting Water e- source for PS II Complex associated with PS II uses several Mn ions to extract 4 electrons from 2 water molecules. P 680 is rereduced by Tyrosine in the PSII reaction center Tyrosine radical is rerreduced by increasing oxidation state of a Mn cluster When Mn cluster reaches +4 state, it can grab 4 e- from 2 HO