CH 33 NAg I and H 3 NAg


- Slides: 13

(CH 3)3 N···Ag. I and H 3 N···Ag. I Studied by Broadband Rotational Spectroscopy and ab initio Calculations Dror M. Bittner, Daniel P. Zaleski, Susanna L. Stephens, Nicholas R. Walker, Anthony C. Legon

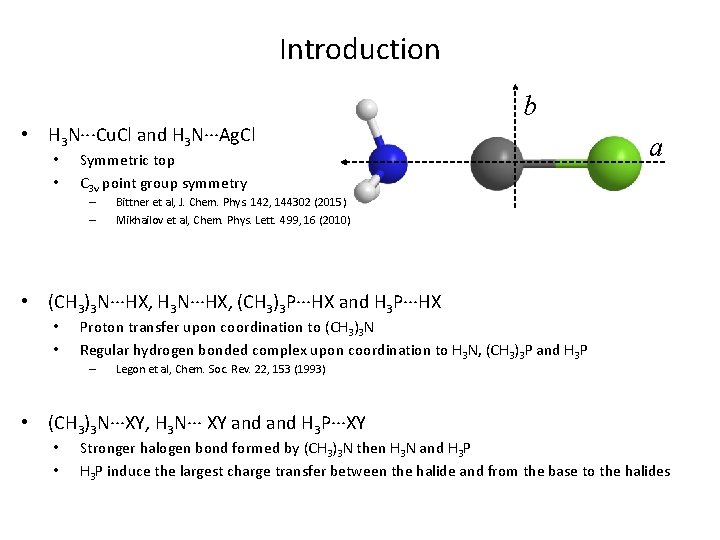
Introduction b • H 3 N···Cu. Cl and H 3 N···Ag. Cl • • Symmetric top C 3 v point group symmetry – – a Bittner et al, J. Chem. Phys. 142, 144302 (2015) Mikhailov et al, Chem. Phys. Lett. 499, 16 (2010) • (CH 3)3 N···HX, H 3 N···HX, (CH 3)3 P···HX and H 3 P···HX • • Proton transfer upon coordination to (CH 3)3 N Regular hydrogen bonded complex upon coordination to H 3 N, (CH 3)3 P and H 3 P – Legon et al, Chem. Soc. Rev. 22, 153 (1993) • (CH 3)3 N···XY, H 3 N··· XY and H 3 P···XY • • Stronger halogen bond formed by (CH 3)3 N then H 3 N and H 3 P induce the largest charge transfer between the halide and from the base to the halides

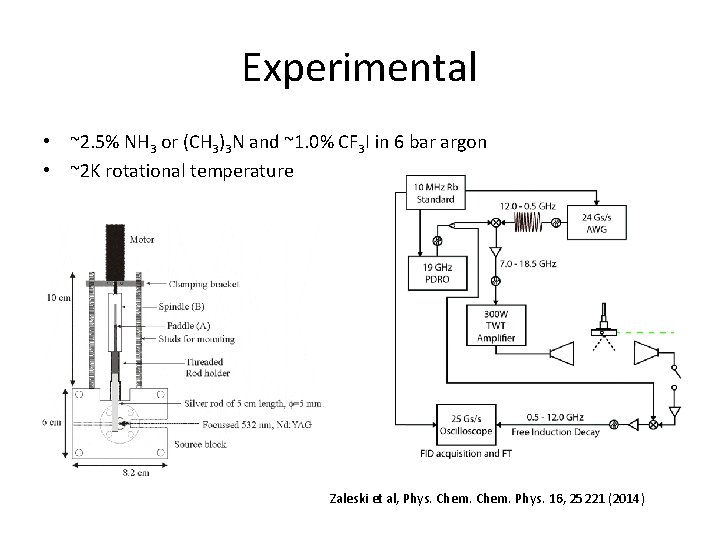
Experimental • ~2. 5% NH 3 or (CH 3)3 N and ~1. 0% CF 3 I in 6 bar argon • ~2 K rotational temperature Zaleski et al, Phys. Chem. Phys. 16, 25221 (2014)

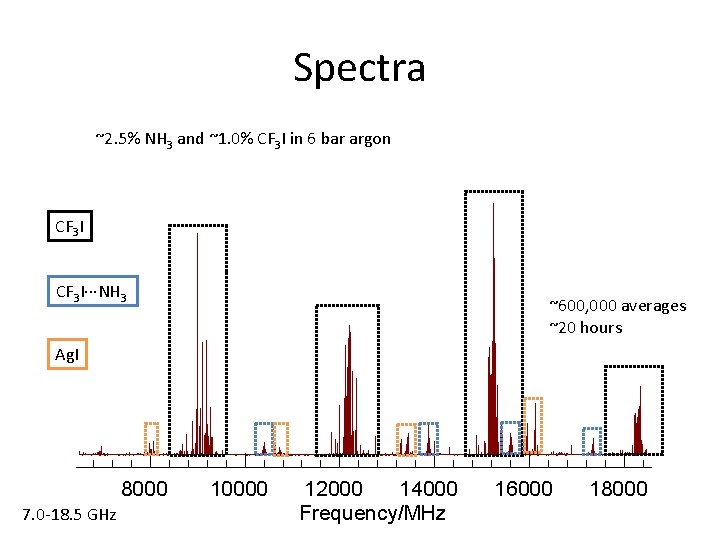
Spectra ~2. 5% NH 3 and ~1. 0% CF 3 I in 6 bar argon CF 3 I···NH 3 ~600, 000 averages ~20 hours Ag. I 8000 7. 0 -18. 5 GHz 10000 12000 14000 Frequency/MHz 16000 18000

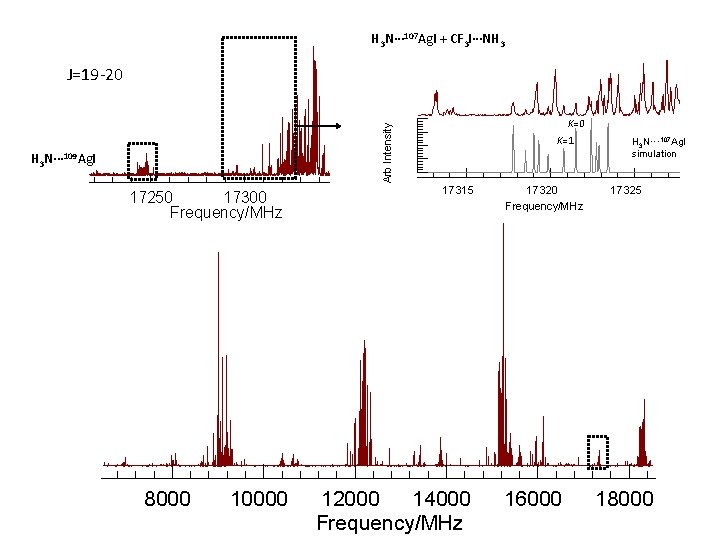
H 3 N··· 107 Ag. I + CF 3 I···NH 3 J=19 -20 Arb Intensity K=0 H 3 N··· 109 Ag. I 17250 17300 Frequency/MHz 8000 10000 K=1 17315 12000 14000 Frequency/MHz 17320 Frequency/MHz 16000 H 3 N··· 107 Ag. I simulation 17325 18000

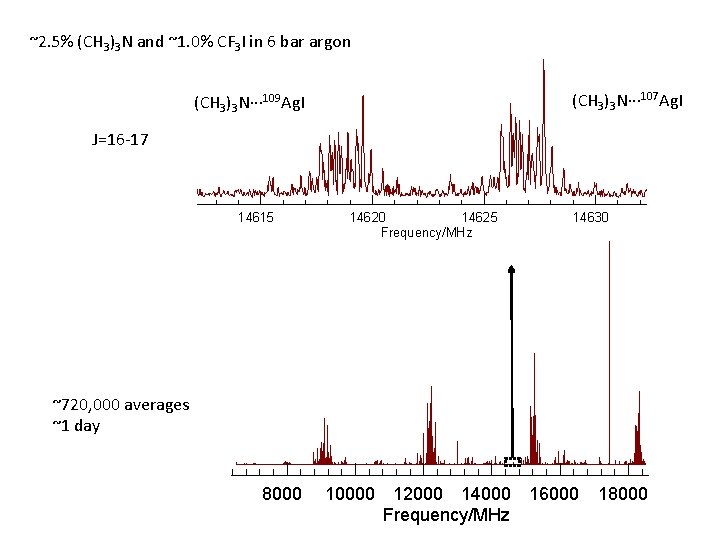
~2. 5% (CH 3)3 N and ~1. 0% CF 3 I in 6 bar argon (CH 3)3 N··· 107 Ag. I (CH 3)3 N··· 109 Ag. I J=16 -17 14615 14620 14625 Frequency/MHz 14630 ~720, 000 averages ~1 day 8000 10000 12000 14000 Frequency/MHz 16000 18000

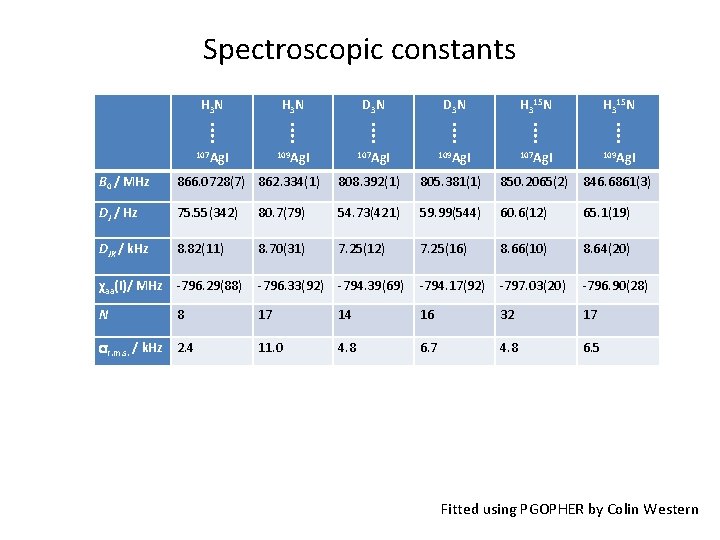
Spectroscopic constants H 3 N D 3 N H 315 N ⁞ ⁞ ⁞ 107 Ag. I 109 Ag. I B 0 / MHz 866. 0728(7) 862. 334(1) 808. 392(1) 805. 381(1) 850. 2065(2) 846. 6861(3) DJ / Hz 75. 55(342) 80. 7(79) 54. 73(421) 59. 99(544) 60. 6(12) 65. 1(19) DJK / k. Hz 8. 82(11) 8. 70(31) 7. 25(12) 7. 25(16) 8. 66(10) 8. 64(20) χaa(I)/ MHz -796. 29(88) -796. 33(92) -794. 39(69) -794. 17(92) -797. 03(20) -796. 90(28) N 8 17 14 16 32 17 r. m. s. / k. Hz 2. 4 11. 0 4. 8 6. 7 4. 8 6. 5 Fitted using PGOPHER by Colin Western

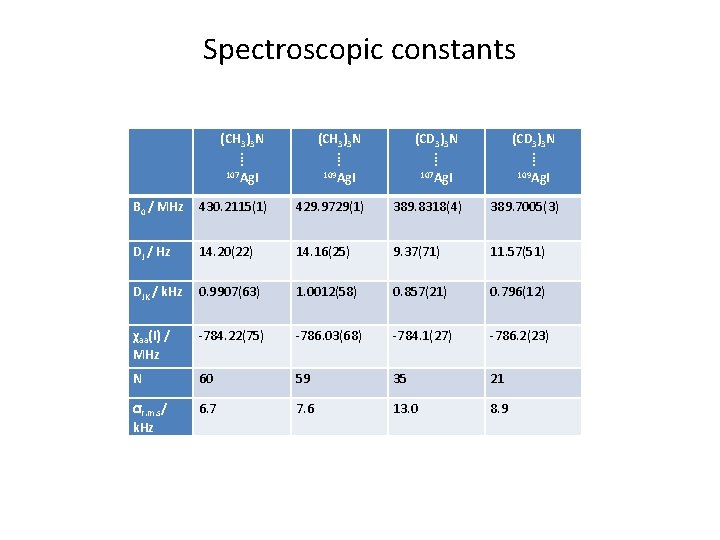
Spectroscopic constants (CH 3)3 N ⁞ 107 Ag. I (CH 3)3 N ⁞ 109 Ag. I (CD 3)3 N ⁞ 107 Ag. I (CD 3)3 N ⁞ 109 Ag. I B 0 / MHz 430. 2115(1) 429. 9729(1) 389. 8318(4) 389. 7005(3) DJ / Hz 14. 20(22) 14. 16(25) 9. 37(71) 11. 57(51) DJK / k. Hz 0. 9907(63) 1. 0012(58) 0. 857(21) 0. 796(12) χaa(I) / MHz -784. 22(75) -786. 03(68) -784. 1(27) -786. 2(23) N 60 59 35 21 r. m. s/ k. Hz 6. 7 7. 6 13. 0 8. 9

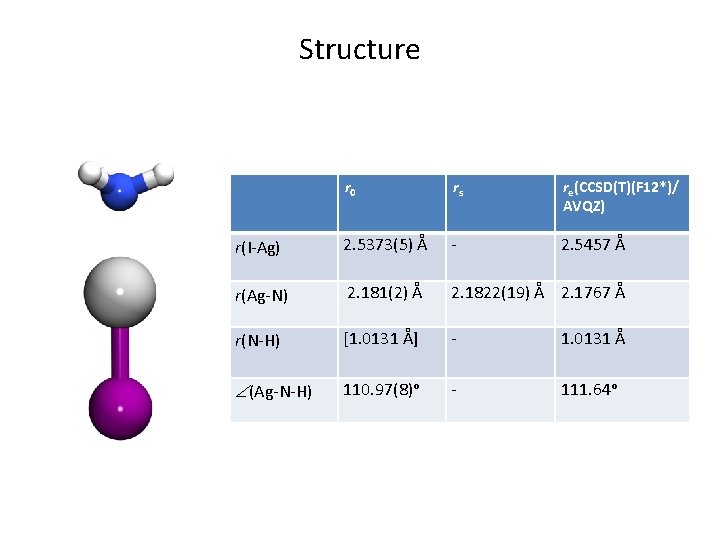
Structure r 0 rs re(CCSD(T)(F 12*)/ AVQZ) r(I-Ag) 2. 5373(5) Å - 2. 5457 Å r(Ag-N) 2. 181(2) Å 2. 1822(19) Å 2. 1767 Å r(N-H) [1. 0131 Å] - 1. 0131 Å (Ag-N-H) 110. 97(8)ᵒ - 111. 64ᵒ

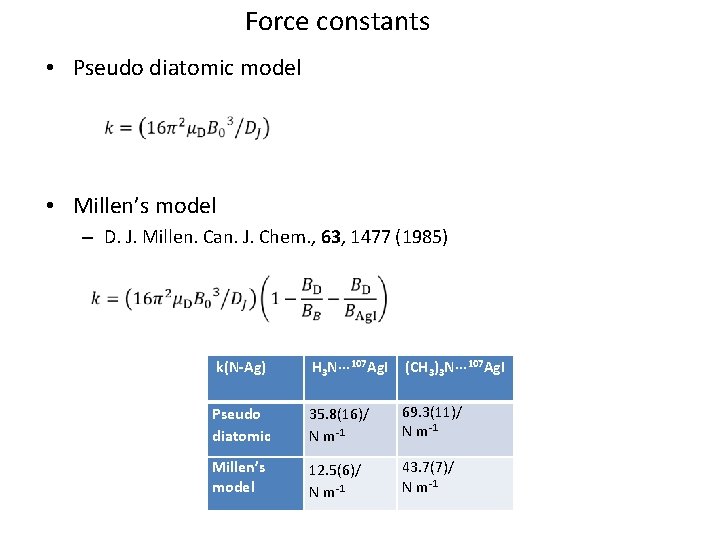
Force constants • Pseudo diatomic model • Millen’s model – D. J. Millen. Can. J. Chem. , 63, 1477 (1985) k(N-Ag) H 3 N··· 107 Ag. I (CH 3)3 N··· 107 Ag. I Pseudo diatomic 35. 8(16)/ N m-1 69. 3(11)/ N m-1 Millen’s model 12. 5(6)/ N m-1 43. 7(7)/ N m-1

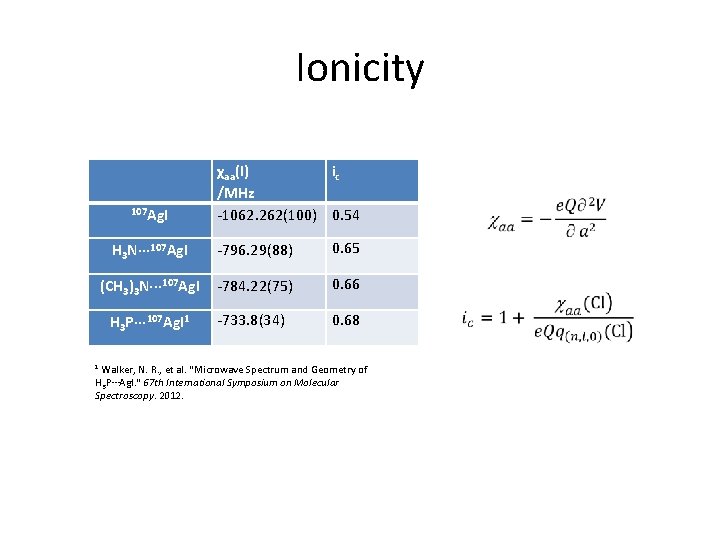
Ionicity 107 Ag. I χaa(I) ic /MHz -1062. 262(100) 0. 54 H 3 N··· 107 Ag. I -796. 29(88) 0. 65 (CH 3)3 N··· 107 Ag. I -784. 22(75) 0. 66 H 3 P··· 107 Ag. I 1 -733. 8(34) 0. 68 Walker, N. R. , et al. "Microwave Spectrum and Geometry of H 3 P···Ag. I. " 67 th International Symposium on Molecular Spectroscopy. 2012. 1

Summary • The rotational spectra of H 3 N···Ag. I and (CH 3) 3 N···Ag. I were fitted to a symmetric top Hamiltonian • Relative intensities of K=0, 1, 2 in H 3 N···Ag. I suggest a C 3 v point group symmetry • The force constant was found to be stronger in (CH 3) 3 N···Ag. I than H 3 N···Ag. I and induce a larger change transfer to the iodine

Acknowledgments The Group: Nick Walker Daniel Zaleski John Mullaney Tony Legon (University of Bristol) Susanna Stephens (University of Manitoba) David Tew (University of Bristol)
 Palimbagan ng el filibusterismo
Palimbagan ng el filibusterismo Tawag sa pag-aaral ng mga tunog ng wika
Tawag sa pag-aaral ng mga tunog ng wika Negosyong pantahanan at pampamayanan
Negosyong pantahanan at pampamayanan Pagsulat ng wakas na pananaliksik
Pagsulat ng wakas na pananaliksik Layunin ng #infairness
Layunin ng #infairness Ano ang kakana
Ano ang kakana Pag aalsa sa tayabas quezon
Pag aalsa sa tayabas quezon Nag hammadi kódex magyarul
Nag hammadi kódex magyarul Nag hammadi
Nag hammadi Pano nagsimula ang wika
Pano nagsimula ang wika Mga makabayang pilipino
Mga makabayang pilipino Mga batayang kaalaman sa ponolohiya
Mga batayang kaalaman sa ponolohiya Ano ang mga katangian ng epiko
Ano ang mga katangian ng epiko Pamana ng indus
Pamana ng indus