Cellular Transport Passive Transport Simple Diffusion Osmosis There









- Slides: 38

Cellular Transport Passive Transport: Simple Diffusion & Osmosis

There are two ways that materials move into & out of the cell membrane: 1. PASSIVE TRANSPORT– does not use energy Examples: simple diffusion osmosis facilitated diffusion 2. ACTIVE TRANSPORT– requires energy (ATP) Examples: endocytosis exocytosis

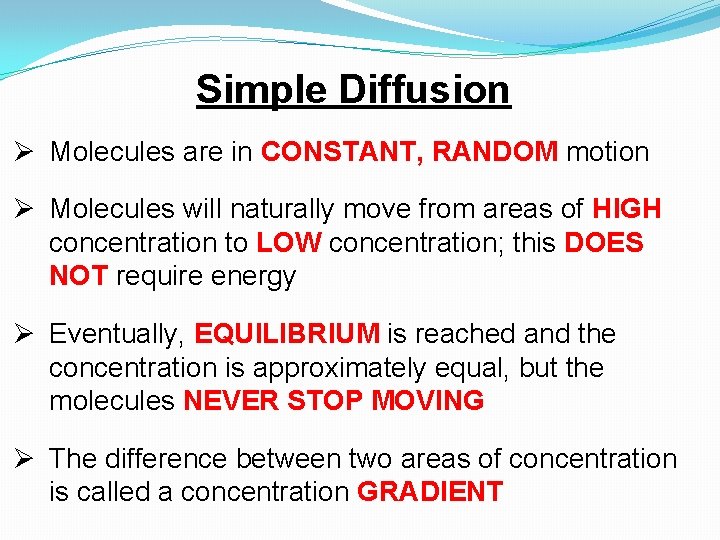
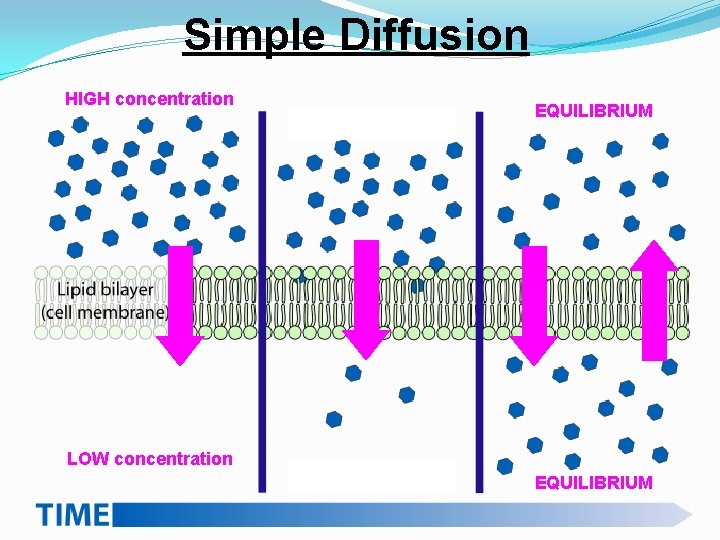
Simple Diffusion Ø Molecules are in CONSTANT, RANDOM motion Ø Molecules will naturally move from areas of HIGH concentration to LOW concentration; this DOES NOT require energy Ø Eventually, EQUILIBRIUM is reached and the concentration is approximately equal, but the molecules NEVER STOP MOVING Ø The difference between two areas of concentration is called a concentration GRADIENT

Simple Diffusion HIGH concentration EQUILIBRIUM LOW concentration EQUILIBRIUM

Example of simple diffusion: If you spray air freshener in one corner of a room, it will eventually diffuse to all parts of the room, because of the random collision of air molecules. (click on picture below for demo) Question: Will the scent spread quicker in warm air or cold air and WHY? WARM air, because the air and perfume molecules are moving faster, meaning they collide more often.

http: //www. wiley. com/legacy/college/boyer/0470003790/a nimations/membrane_transport. htm

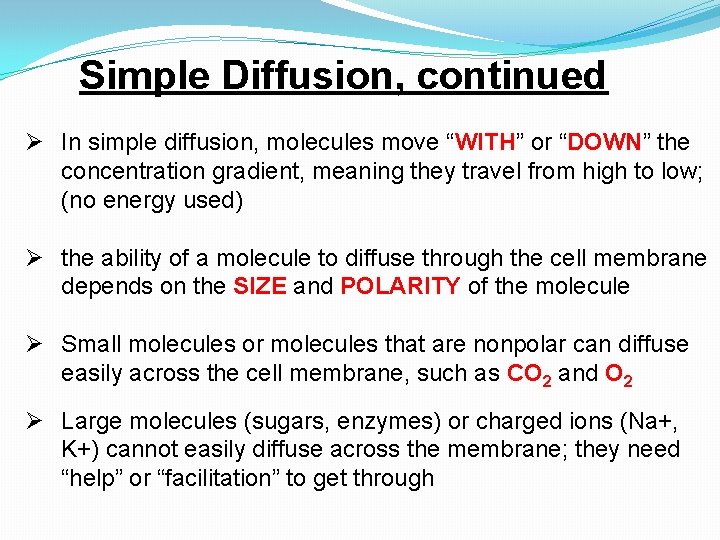
Simple Diffusion, continued Ø In simple diffusion, molecules move “WITH” or “DOWN” the concentration gradient, meaning they travel from high to low; (no energy used) Ø the ability of a molecule to diffuse through the cell membrane depends on the SIZE and POLARITY of the molecule Ø Small molecules or molecules that are nonpolar can diffuse easily across the cell membrane, such as CO 2 and O 2 Ø Large molecules (sugars, enzymes) or charged ions (Na+, K+) cannot easily diffuse across the membrane; they need “help” or “facilitation” to get through


What is Osmosis? • Osmosis is a type of passive transport; no energy is required • Osmosis is the movement of water across a membrane from high to low concentration

The Direction of Osmosis • Water will move in response to the SOLUTE concentration inside the cell and outside the cell. • Examples of solutes: salt, glucose, urea • Water molecules move to where the HIGHEST solute concentration is.

Types of Solutions • HYPERTONIC: there is a HIGHER solute concentration outside the cell – What is the direction of osmosis? Water will move OUT of the cell • HYPOTONIC: there is a LOWER solute concentration outside the cell. – What is the direction of osmosis? Water will move INTO the cell

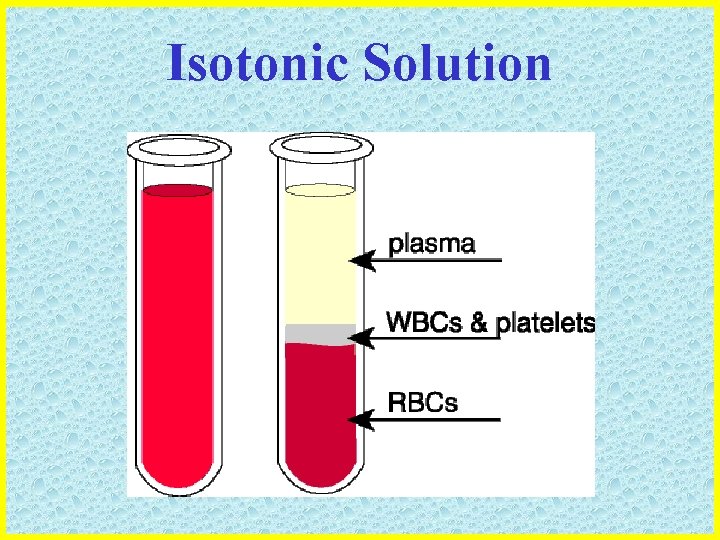
Types of Solutions • ISOTONIC: the solute concentration outside the cell EQUALS the solute concentration inside the cell • Example of an isotonic solution: • BLOOD PLASMA (the clear part of our blood) has the same concentration of solutes as red and white blood cells

Isotonic Solution

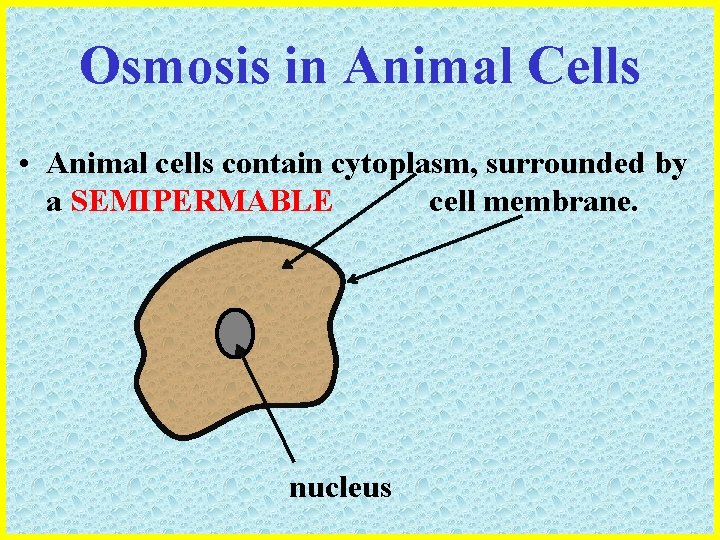
Osmosis in Animal Cells • Animal cells contain cytoplasm, surrounded by a SEMIPERMABLE cell membrane. nucleus

Osmosis Scenario #1 • What would happen to a human skin cell if it were placed into concentrated salt water?

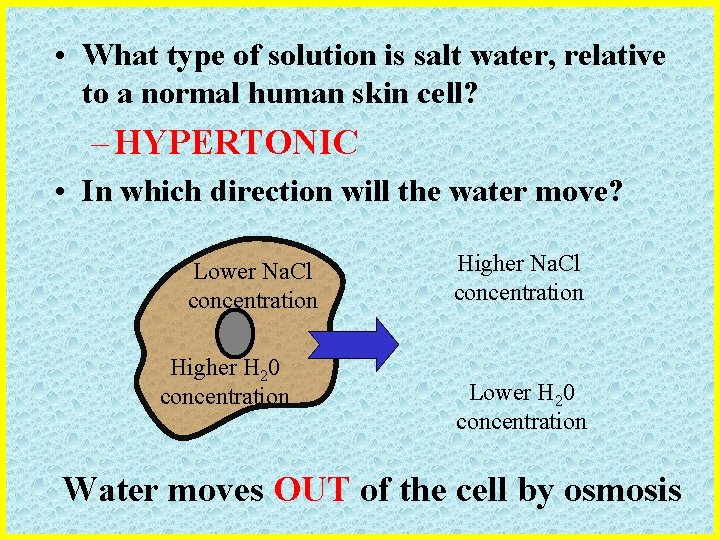
• What type of solution is salt water, relative to a normal human skin cell? – HYPERTONIC • In which direction will the water move? Lower Na. Cl concentration Higher H 20 concentration Higher Na. Cl concentration Lower H 20 concentration Water moves OUT of the cell by osmosis

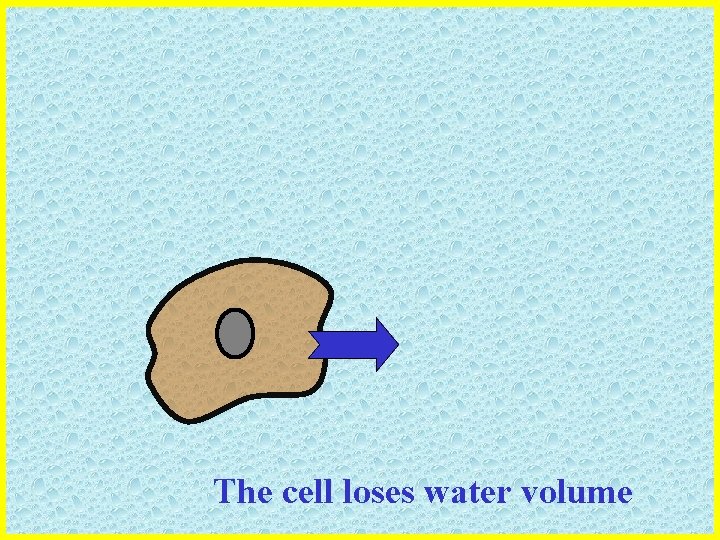
The cell loses water volume

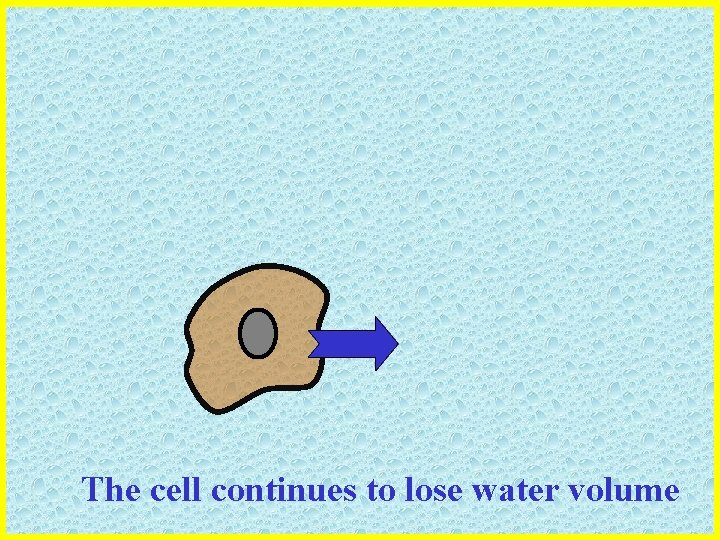
The cell continues to lose water volume

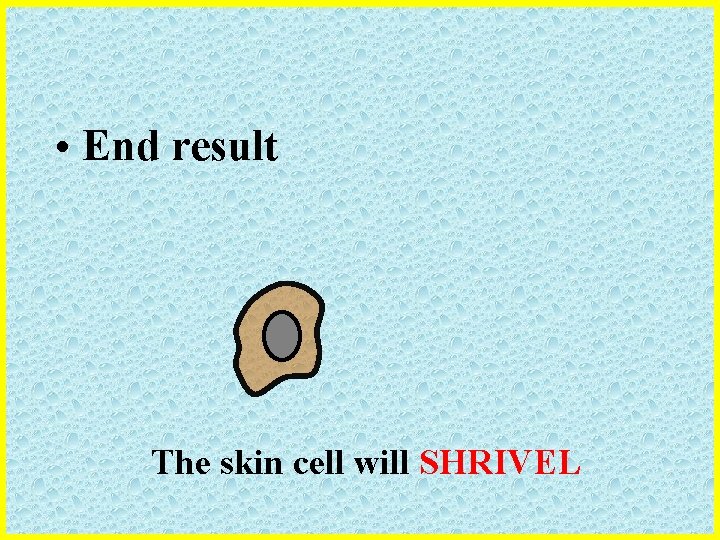
• End result The skin cell will SHRIVEL

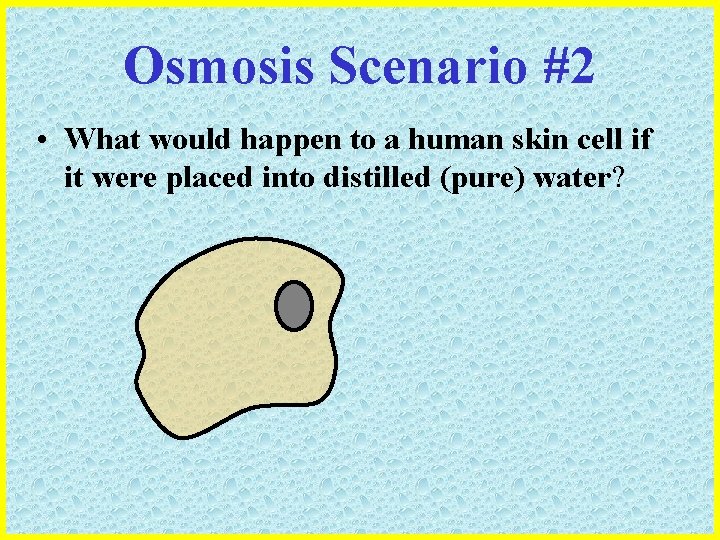
Osmosis Scenario #2 • What would happen to a human skin cell if it were placed into distilled (pure) water?

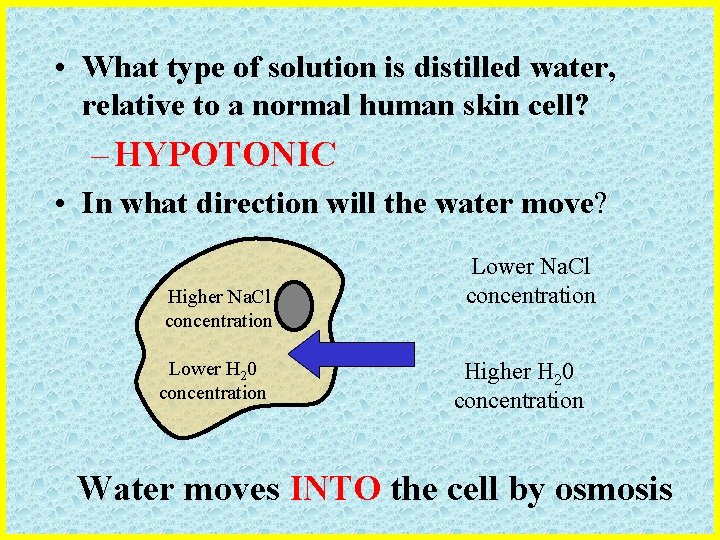
• What type of solution is distilled water, relative to a normal human skin cell? – HYPOTONIC • In what direction will the water move? Higher Na. Cl concentration Lower H 20 concentration Lower Na. Cl concentration Higher H 20 concentration Water moves INTO the cell by osmosis

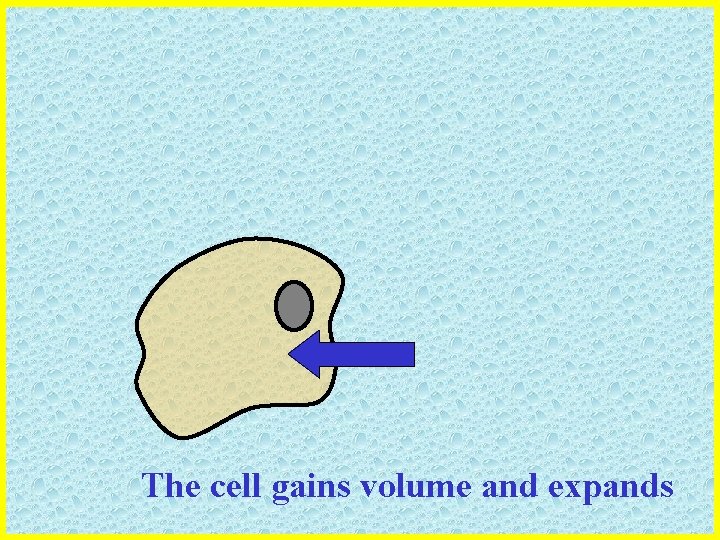
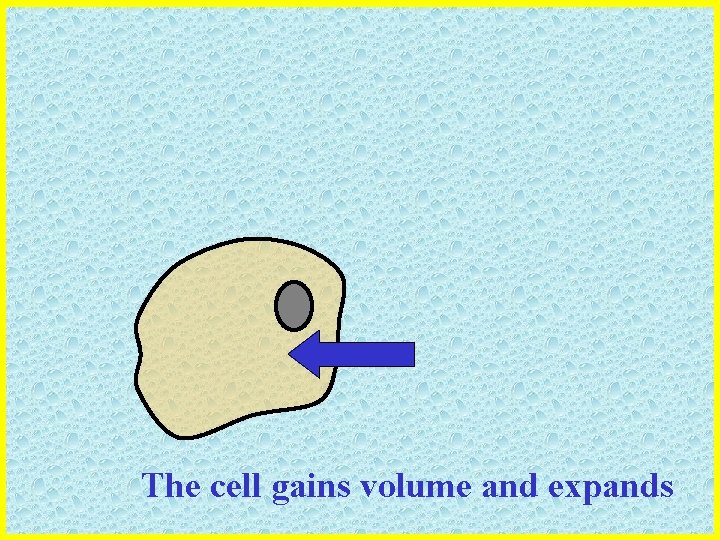
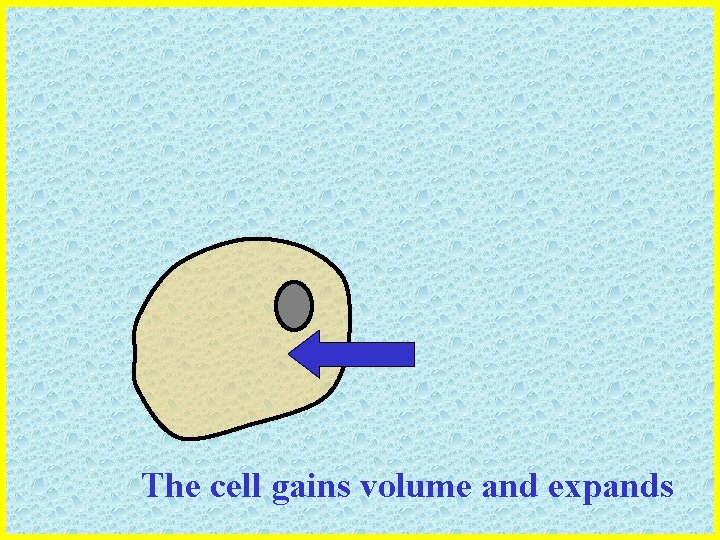
The cell gains volume and expands

The cell gains volume and expands

The cell gains volume and expands

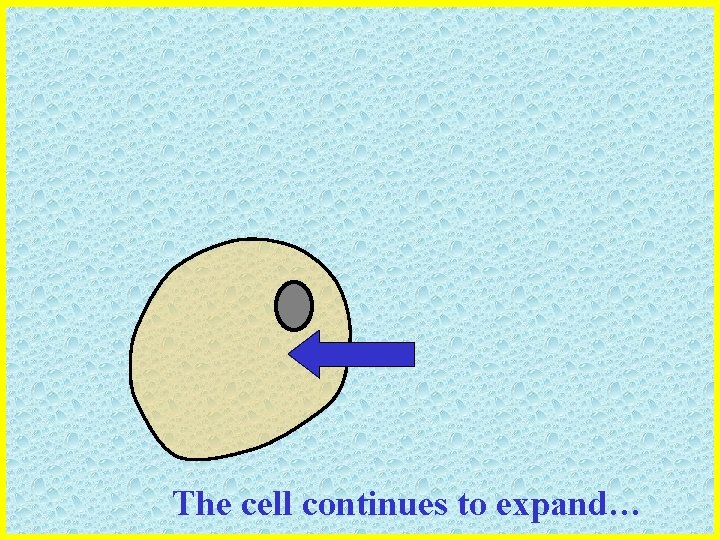
The cell continues to expand…

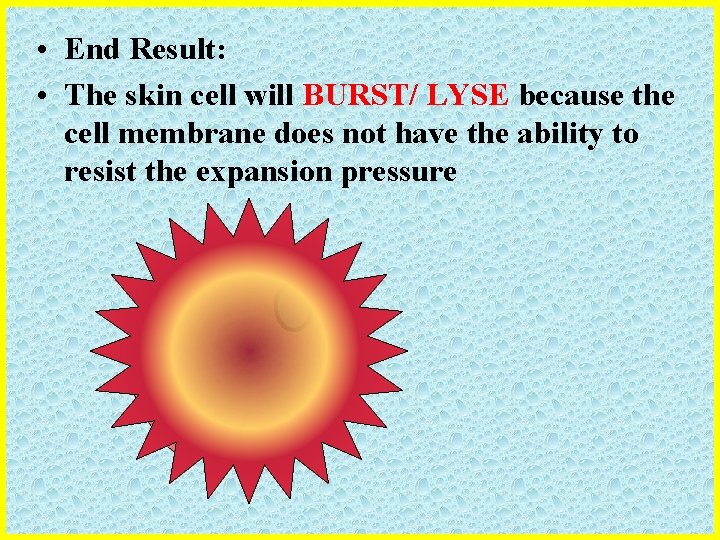
• End Result: • The skin cell will BURST/ LYSE because the cell membrane does not have the ability to resist the expansion pressure

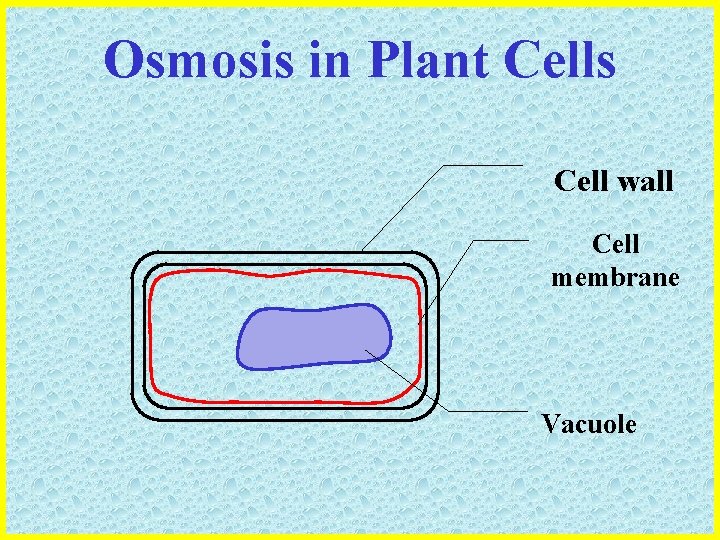
Osmosis in Plant Cells • Plant cells are surrounded by a cell wall composed of CELLULOSE which… – Is freely permeable to water – Is inelastic – Able to resist cell expansion • Plant cells also have a large VACUOLE which… – Contains a solution of salt, sugars and other ions – Is bound by a selectively permeable membrane – Takes in or releases water by osmosis

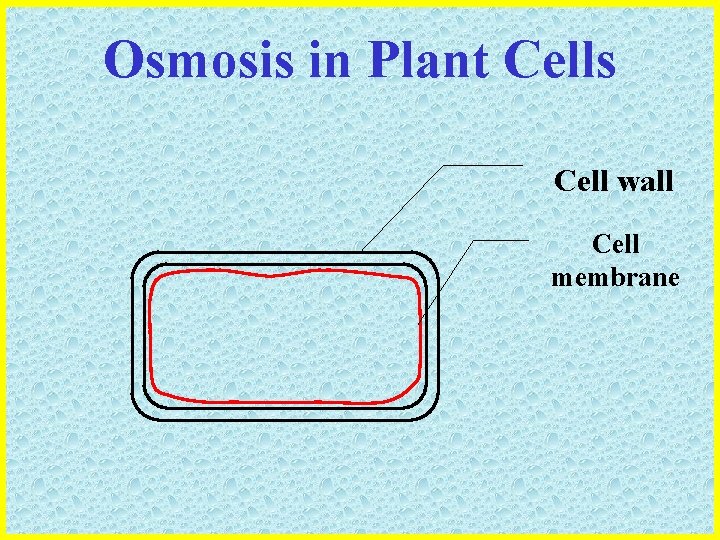
Osmosis in Plant Cells Cell wall

Osmosis in Plant Cells Cell wall Cell membrane

Osmosis in Plant Cells Cell wall Cell membrane Vacuole

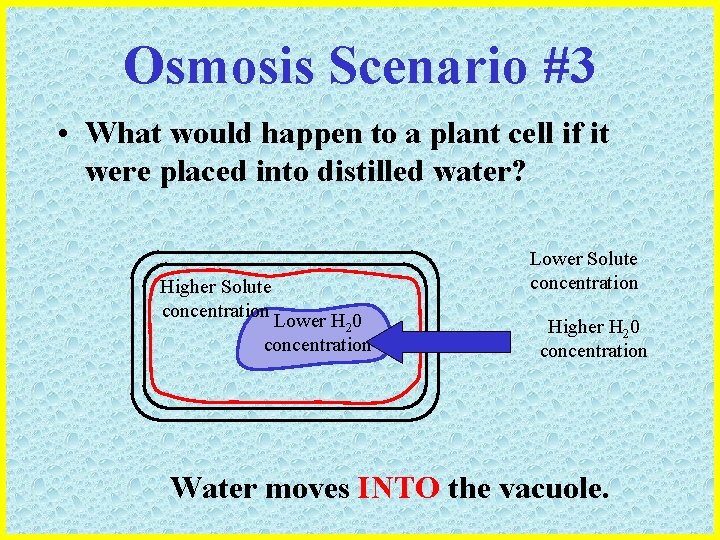
Osmosis Scenario #3 • What would happen to a plant cell if it were placed into distilled water? Higher Solute concentration Lower H 20 concentration Lower Solute concentration Higher H 20 concentration Water moves INTO the vacuole.

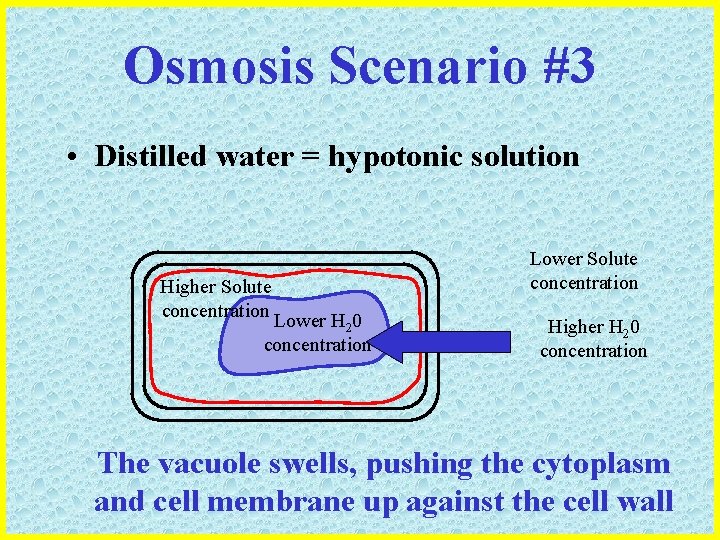
Osmosis Scenario #3 • Distilled water = hypotonic solution Higher Solute concentration Lower H 20 concentration Lower Solute concentration Higher H 20 concentration The vacuole swells, pushing the cytoplasm and cell membrane up against the cell wall

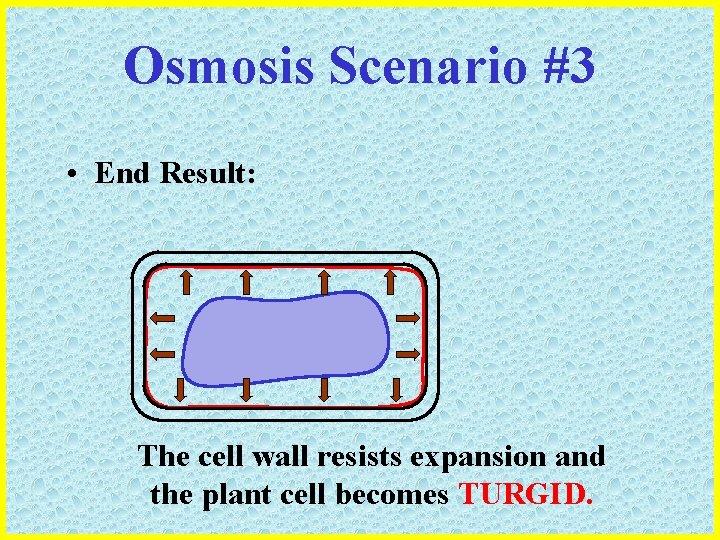
Osmosis Scenario #3 • End Result: The cell wall resists expansion and the plant cell becomes TURGID.

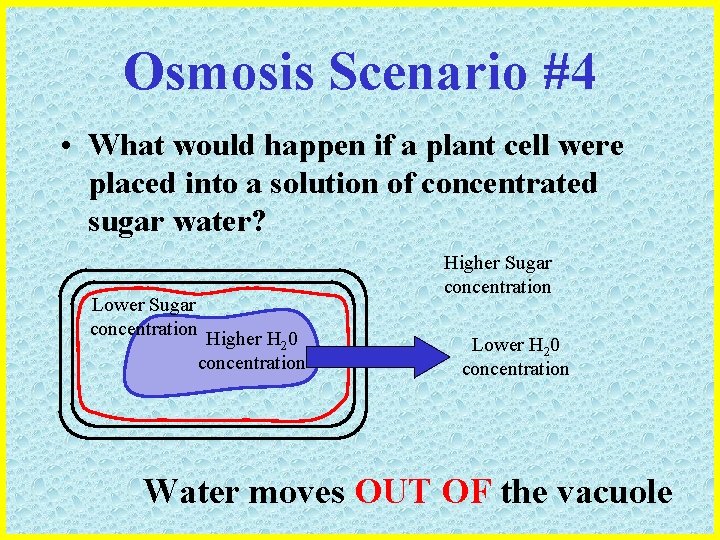
Osmosis Scenario #4 • What would happen if a plant cell were placed into a solution of concentrated sugar water? Lower Sugar concentration Higher H 20 concentration Higher Sugar concentration Lower H 20 concentration Water moves OUT OF the vacuole

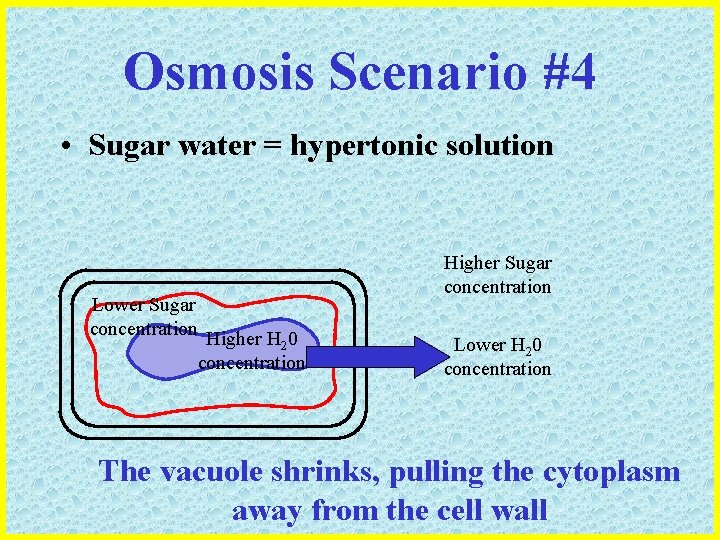
Osmosis Scenario #4 • Sugar water = hypertonic solution Lower Sugar concentration Higher H 20 concentration Higher Sugar concentration Lower H 20 concentration The vacuole shrinks, pulling the cytoplasm away from the cell wall

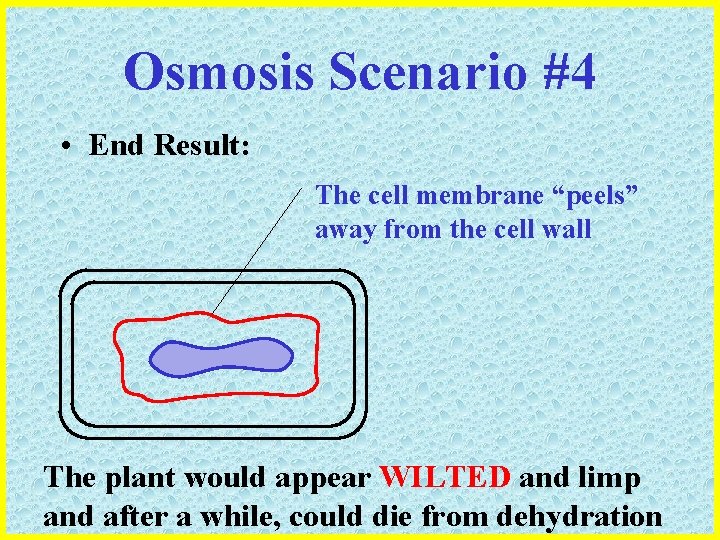
Osmosis Scenario #4 • End Result: The cell membrane “peels” away from the cell wall The plant would appear WILTED and limp and after a while, could die from dehydration

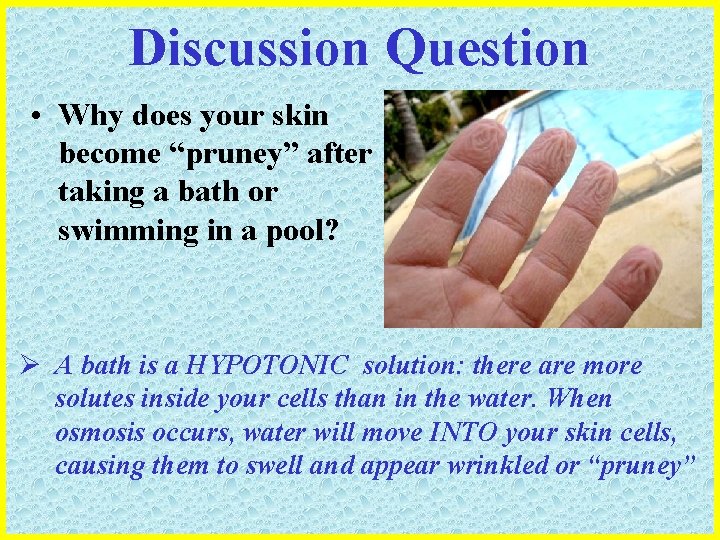
Discussion Question • Why does your skin become “pruney” after taking a bath or swimming in a pool? Ø A bath is a HYPOTONIC solution: there are more solutes inside your cells than in the water. When osmosis occurs, water will move INTO your skin cells, causing them to swell and appear wrinkled or “pruney”
