CDRHs TPLC Reorganization Impact on Cardiovascular Devices Matthew




- Slides: 9

CDRH’s TPLC Reorganization: Impact on Cardiovascular Devices Matthew Hillebrenner Division of Cardiovascular Devices Center for Devices and Radiological Health U. S. Food and Drug Administration March 6, 2018

Disclosures I have no relevant financial relationships

TPLC Reorganization Goals • Create an agile infrastructure that can adapt to future organizational, regulatory, and scientific needs. • Facilitate information-sharing to help make better informed decisions. • Facilitate professional development for all employees by increasing opportunities for cross-skills development and creating multifunctional positions. 3

TPLC Reorganization Goals • Ensure process and policy consistency. • Minimize organizational layers of review and facilitate employee professional development, to achieve more efficient work processes and allow employees to leverage their knowledge of pre- and post-market information to optimize decision-making. • Allow the increases in efficiency and organizational flexibility to translate into reasonable employee workloads, so that managers and staff can have healthy work-life balances. 4

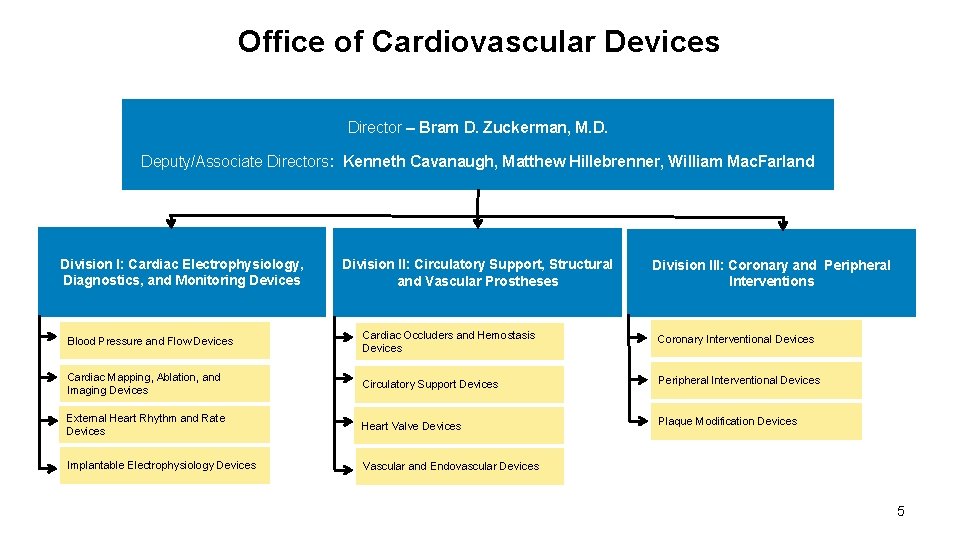
Office of Cardiovascular Devices Director – Bram D. Zuckerman, M. D. Deputy/Associate Directors: Kenneth Cavanaugh, Matthew Hillebrenner, William Mac. Farland Division I: Cardiac Electrophysiology, Diagnostics, and Monitoring Devices Division II: Circulatory Support, Structural and Vascular Prostheses Division III: Coronary and Peripheral Interventions Blood Pressure and Flow Devices Cardiac Occluders and Hemostasis Devices Coronary Interventional Devices Cardiac Mapping, Ablation, and Imaging Devices Circulatory Support Devices Peripheral Interventional Devices External Heart Rhythm and Rate Devices Heart Valve Devices Plaque Modification Devices Implantable Electrophysiology Devices Vascular and Endovascular Devices 5

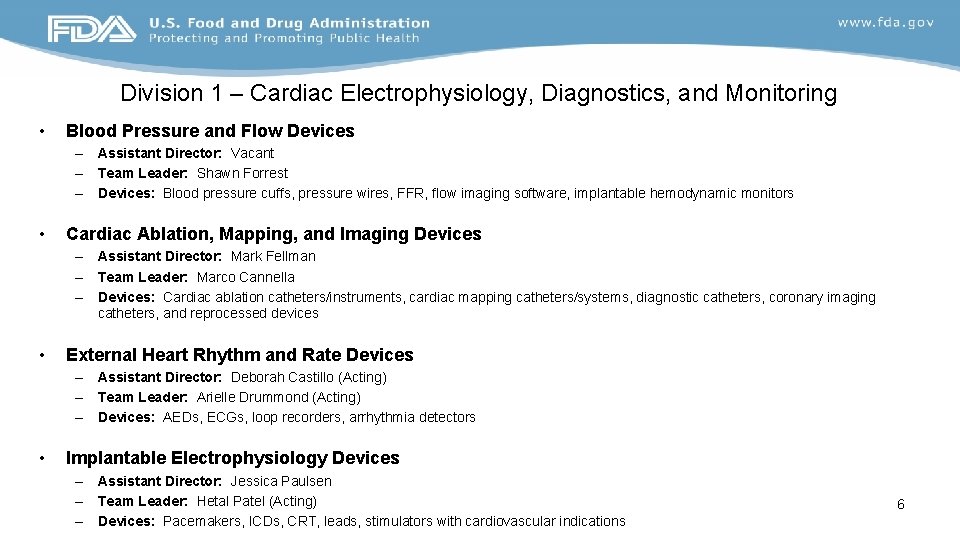
Division 1 – Cardiac Electrophysiology, Diagnostics, and Monitoring • Blood Pressure and Flow Devices – Assistant Director: Vacant – Team Leader: Shawn Forrest – Devices: Blood pressure cuffs, pressure wires, FFR, flow imaging software, implantable hemodynamic monitors • Cardiac Ablation, Mapping, and Imaging Devices – Assistant Director: Mark Fellman – Team Leader: Marco Cannella – Devices: Cardiac ablation catheters/instruments, cardiac mapping catheters/systems, diagnostic catheters, coronary imaging catheters, and reprocessed devices • External Heart Rhythm and Rate Devices – Assistant Director: Deborah Castillo (Acting) – Team Leader: Arielle Drummond (Acting) – Devices: AEDs, ECGs, loop recorders, arrhythmia detectors • Implantable Electrophysiology Devices – Assistant Director: Jessica Paulsen – Team Leader: Hetal Patel (Acting) – Devices: Pacemakers, ICDs, CRT, leads, stimulators with cardiovascular indications 6

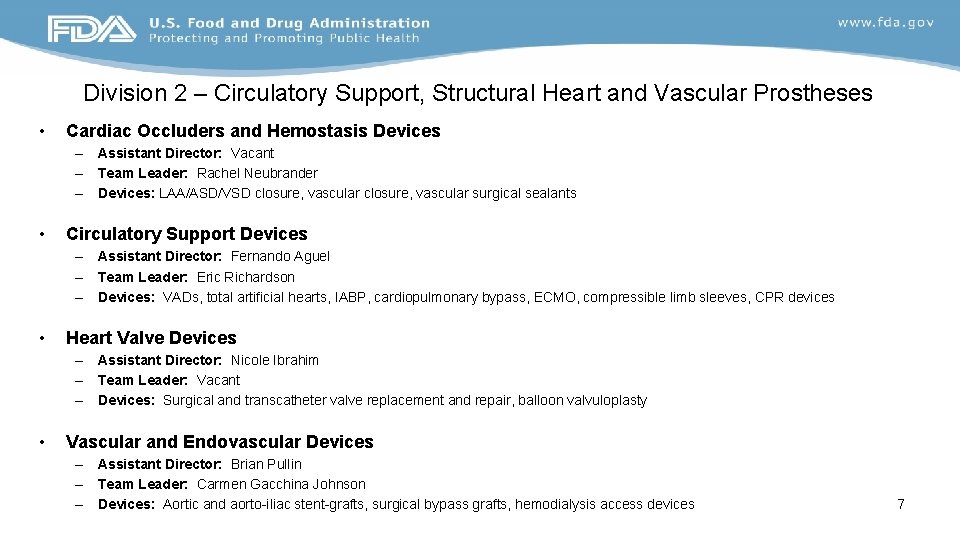
Division 2 – Circulatory Support, Structural Heart and Vascular Prostheses • Cardiac Occluders and Hemostasis Devices – Assistant Director: Vacant – Team Leader: Rachel Neubrander – Devices: LAA/ASD/VSD closure, vascular surgical sealants • Circulatory Support Devices – Assistant Director: Fernando Aguel – Team Leader: Eric Richardson – Devices: VADs, total artificial hearts, IABP, cardiopulmonary bypass, ECMO, compressible limb sleeves, CPR devices • Heart Valve Devices – Assistant Director: Nicole Ibrahim – Team Leader: Vacant – Devices: Surgical and transcatheter valve replacement and repair, balloon valvuloplasty • Vascular and Endovascular Devices – Assistant Director: Brian Pullin – Team Leader: Carmen Gacchina Johnson – Devices: Aortic and aorto-iliac stent-grafts, surgical bypass grafts, hemodialysis access devices 7

Division 3 – Coronary and Peripheral Interventions • Coronary Interventional Devices – Assistant Director: Michael John – Team Leader: Lydia Glaw – Devices: Coronary stents, peripheral/coronary guidewires, support catheters, coronary sinus interventions • Peripheral Interventional Devices – Assistant Director: Misti Malone – Team Leader: Vacant – Devices: Peripheral stents (carotid, femoral, iliac, renal, venous), hypertension treatments, introducer sheaths, embolization devices, peripheral EPD • Plaque Modification Devices – Assistant Director: Gregory O’Connell – Team Leader: Eleni Whatley – Devices: Coronary/peripheral drug-coated and uncoated balloon catheters, atherectomy, embolectomy, thrombectomy, infusion catheters, IVC filters 8

Thank You! matthew. hillebrenner@fda. hhs. gov
















