Catalytic Mechanism of Chymotrypsin slide 1 Chymotrypsin Protease
- Slides: 14

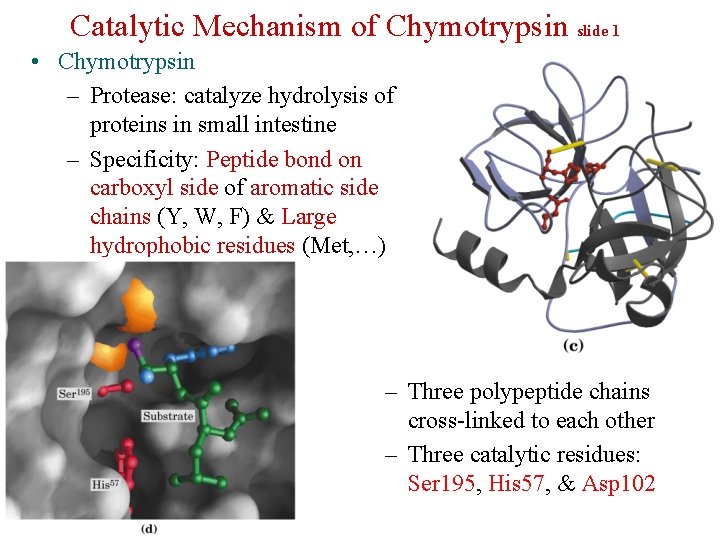
Catalytic Mechanism of Chymotrypsin slide 1 • Chymotrypsin – Protease: catalyze hydrolysis of proteins in small intestine – Specificity: Peptide bond on carboxyl side of aromatic side chains (Y, W, F) & Large hydrophobic residues (Met, …) – Three polypeptide chains cross-linked to each other – Three catalytic residues: Ser 195, His 57, & Asp 102

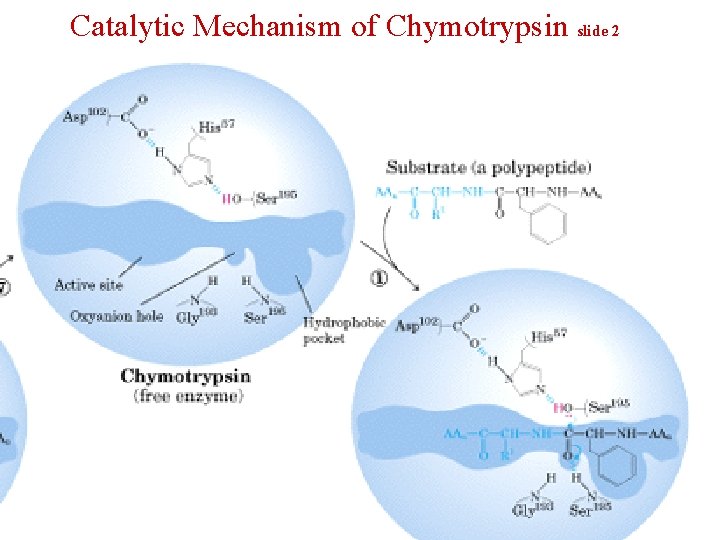
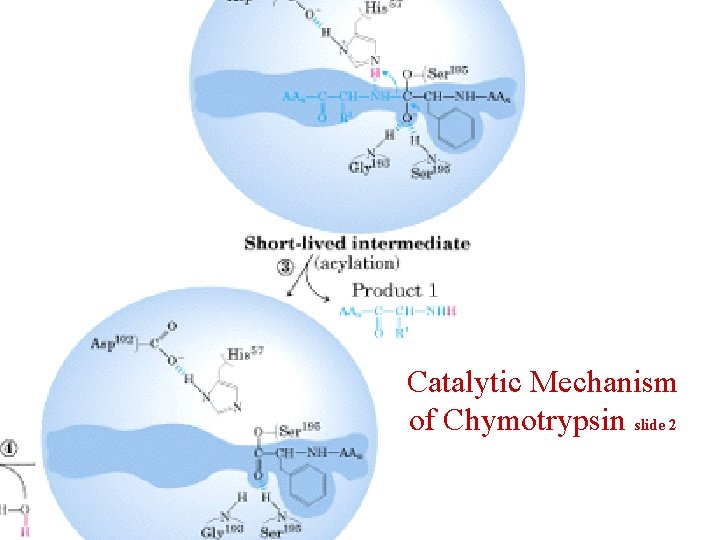
Catalytic Mechanism of Chymotrypsin slide 2

Catalytic Mechanism of Chymotrypsin slide 2

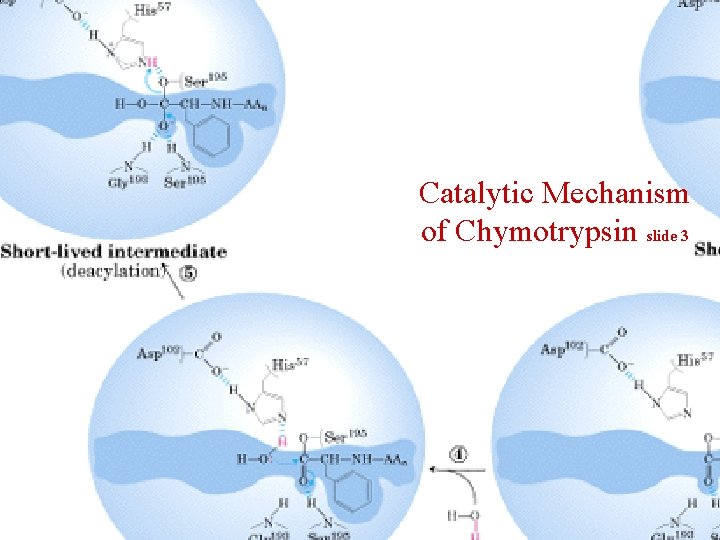
Catalytic Mechanism of Chymotrypsin slide 3

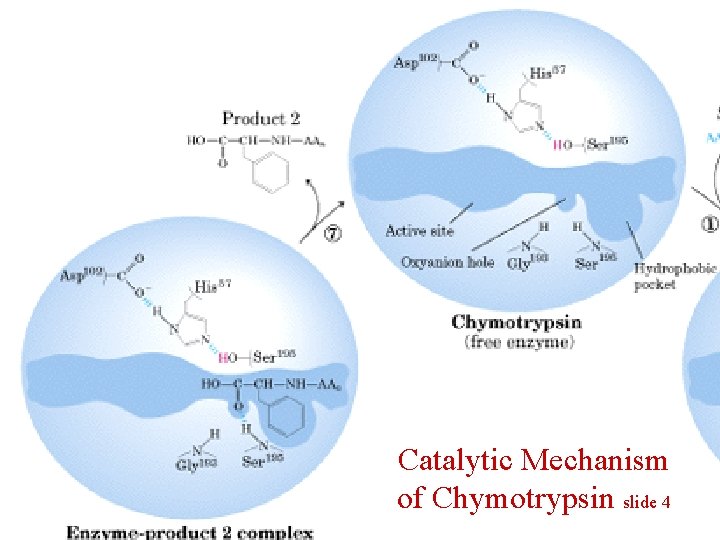
Catalytic Mechanism of Chymotrypsin slide 4

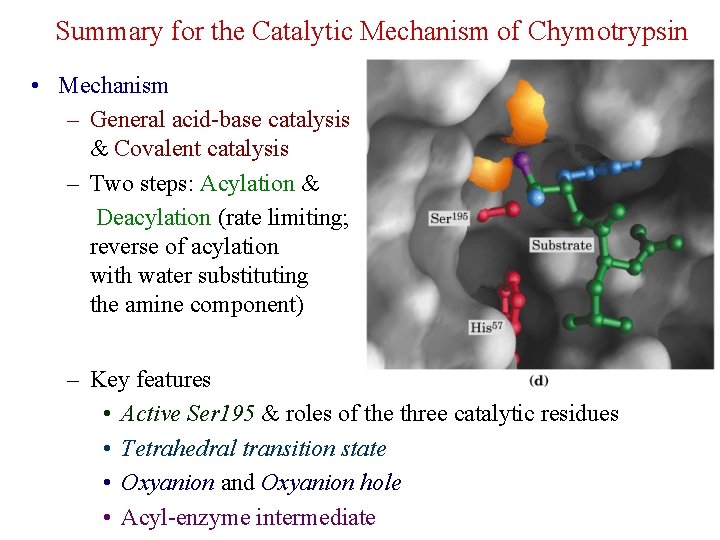
Summary for the Catalytic Mechanism of Chymotrypsin • Mechanism – General acid-base catalysis & Covalent catalysis – Two steps: Acylation & Deacylation (rate limiting; reverse of acylation with water substituting the amine component) – Key features • Active Ser 195 & roles of the three catalytic residues • Tetrahedral transition state • Oxyanion and Oxyanion hole • Acyl-enzyme intermediate

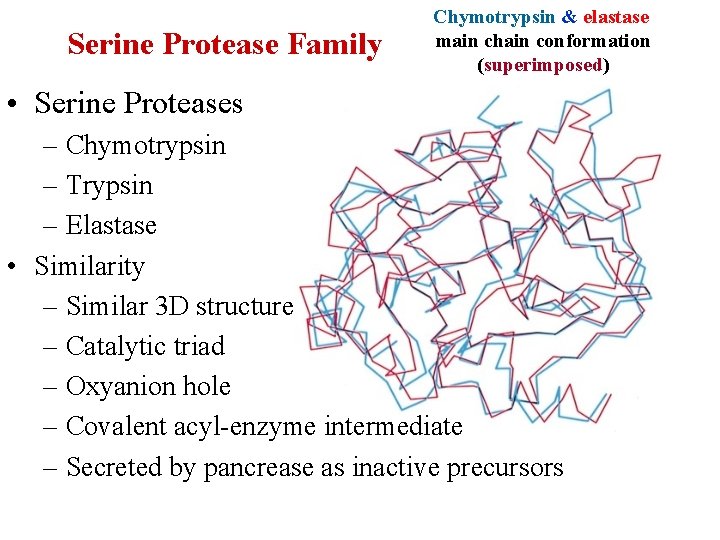
Serine Protease Family Chymotrypsin & elastase main chain conformation (superimposed) • Serine Proteases – Chymotrypsin – Trypsin – Elastase • Similarity – Similar 3 D structure – Catalytic triad – Oxyanion hole – Covalent acyl-enzyme intermediate – Secreted by pancrease as inactive precursors

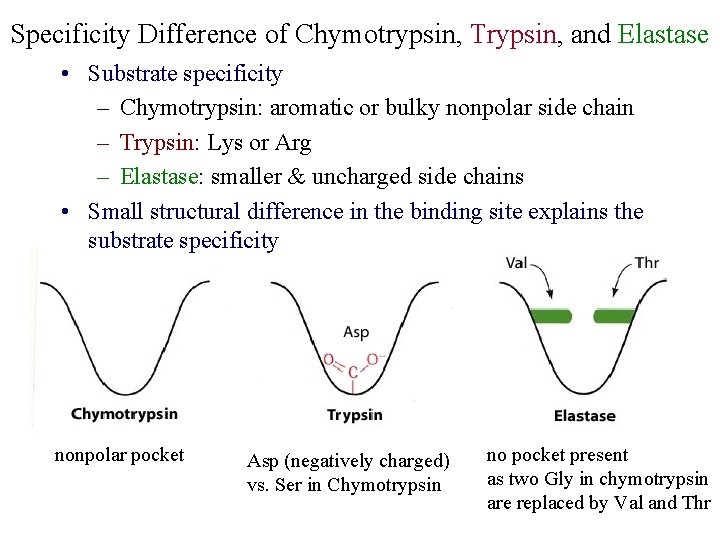
Specificity Difference of Chymotrypsin, Trypsin, and Elastase • Substrate specificity – Chymotrypsin: aromatic or bulky nonpolar side chain – Trypsin: Lys or Arg – Elastase: smaller & uncharged side chains • Small structural difference in the binding site explains the substrate specificity nonpolar pocket Asp (negatively charged) vs. Ser in Chymotrypsin no pocket present as two Gly in chymotrypsin are replaced by Val and Thr

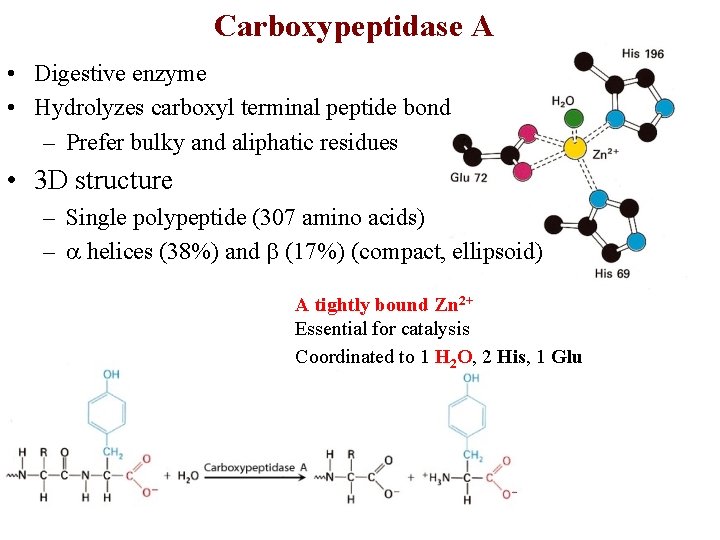
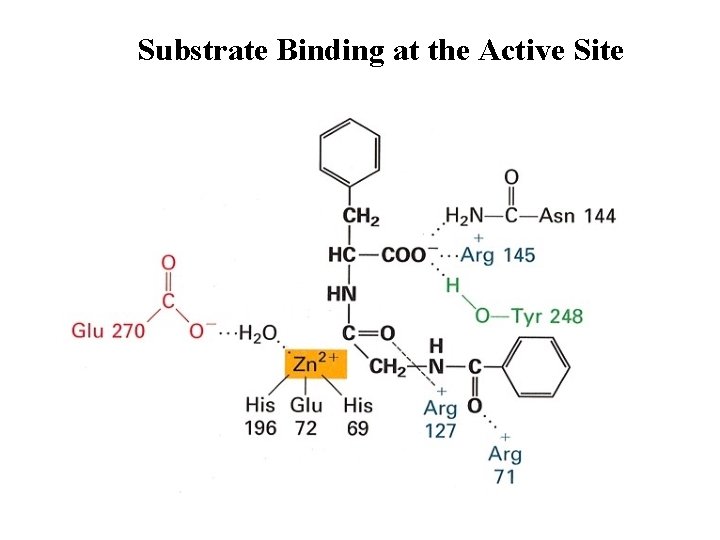
Carboxypeptidase A • Digestive enzyme • Hydrolyzes carboxyl terminal peptide bond – Prefer bulky and aliphatic residues • 3 D structure – Single polypeptide (307 amino acids) – helices (38%) and (17%) (compact, ellipsoid) A tightly bound Zn 2+ Essential for catalysis Coordinated to 1 H 2 O, 2 His, 1 Glu

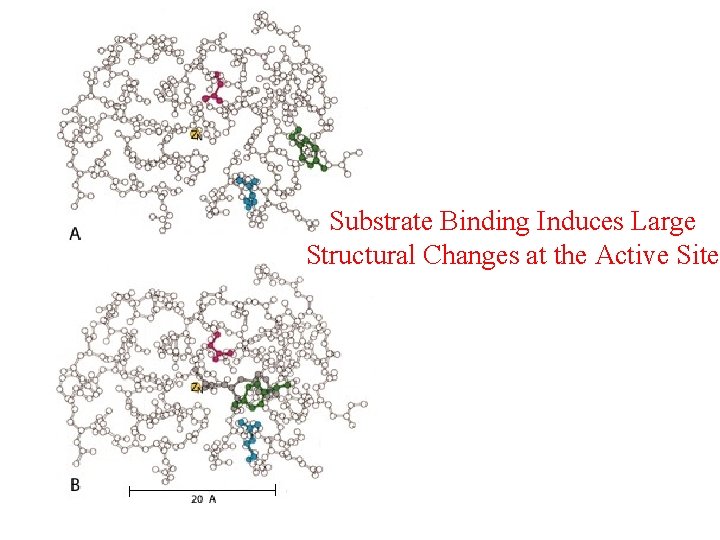
Substrate Binding Induces Large Structural Changes at the Active Site

Substrate Binding Induces Large Structural Changes at the Active Site • 3 D Structure of peptidase A/glycyltyrosine complex – Substrate-induced structural change at active site • 12 Å movement of Tyr 248 -OH & rotation (Moves from surface to substrate terminal COO-) – New interaction: Tyr 248 O H –O C=O – Closes active-site cavity – Extrude water from cavity • Arg 145 moves 2 Å – New interaction: Arg 145 & –O C=O (substrate) • Terminal side chain of substrate – Now sits in a hydrophobic pocket – Induced-fit model (Daniel Koshland, Jr. )

Substrate Binding at the Active Site

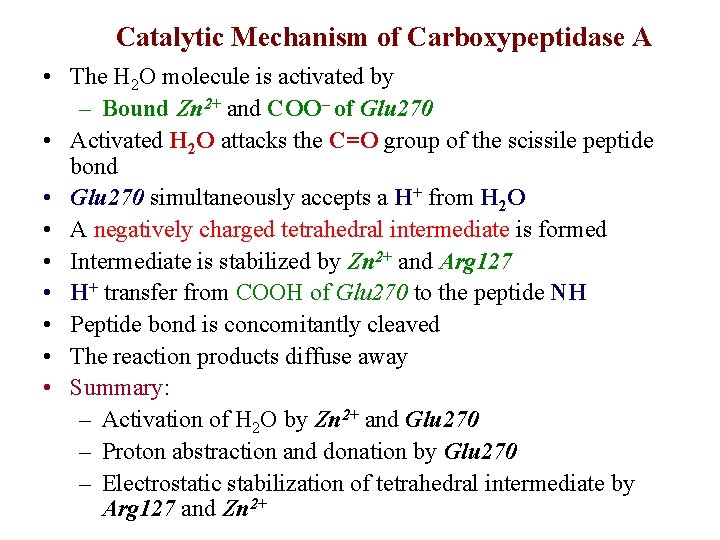
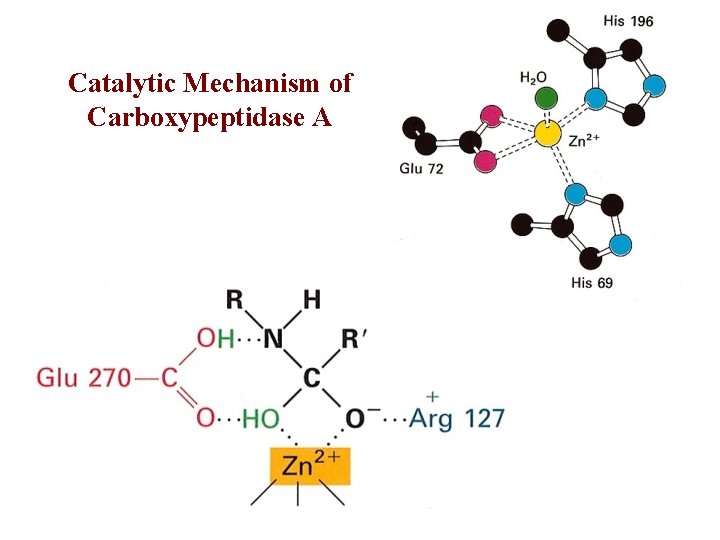
Catalytic Mechanism of Carboxypeptidase A • The H 2 O molecule is activated by – Bound Zn 2+ and COO– of Glu 270 • Activated H 2 O attacks the C=O group of the scissile peptide bond • Glu 270 simultaneously accepts a H+ from H 2 O • A negatively charged tetrahedral intermediate is formed • Intermediate is stabilized by Zn 2+ and Arg 127 • H+ transfer from COOH of Glu 270 to the peptide NH • Peptide bond is concomitantly cleaved • The reaction products diffuse away • Summary: – Activation of H 2 O by Zn 2+ and Glu 270 – Proton abstraction and donation by Glu 270 – Electrostatic stabilization of tetrahedral intermediate by Arg 127 and Zn 2+

Catalytic Mechanism of Carboxypeptidase A