David L Nelson and Michael M Cox LEHNINGER







![kcat = Vmax / [E]total kcat has units of reciprocal time kcat = Vmax / [E]total kcat has units of reciprocal time](https://slidetodoc.com/presentation_image_h/df4a538b8a53ffb9ce2a185a311b7090/image-15.jpg)











- Slides: 46

David L. Nelson and Michael M. Cox LEHNINGER PRINCIPLES OF BIOCHEMISTRY Sixth Edition CHAPTER 6 Enzymes © 2013 W. H. Freeman and Company



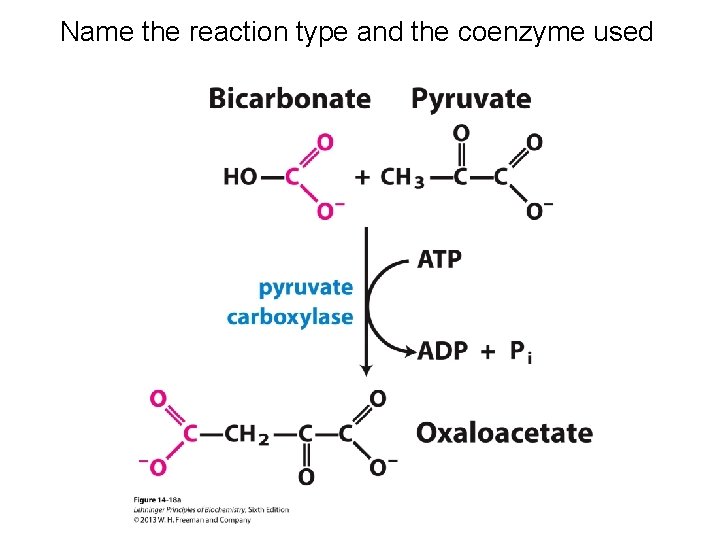
Name the reaction type and the coenzyme used

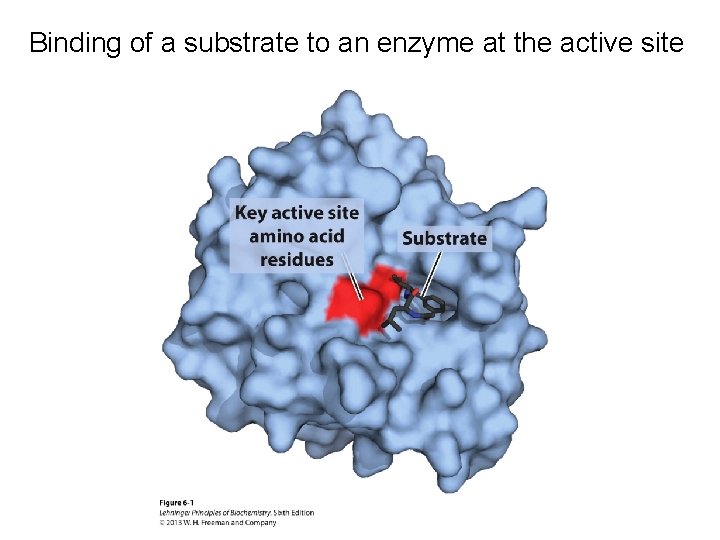
Binding of a substrate to an enzyme at the active site

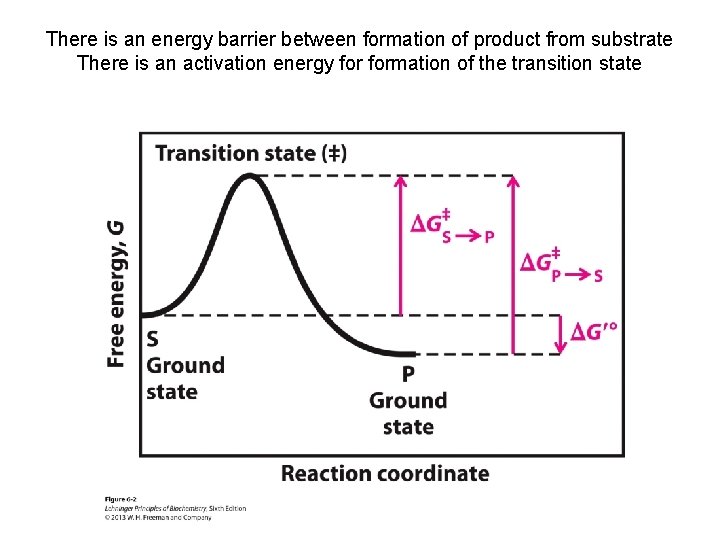
There is an energy barrier between formation of product from substrate There is an activation energy formation of the transition state

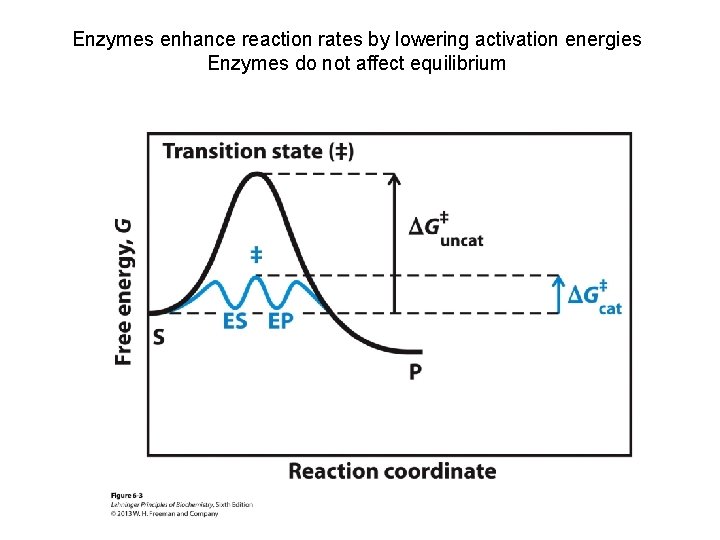
Enzymes enhance reaction rates by lowering activation energies Enzymes do not affect equilibrium




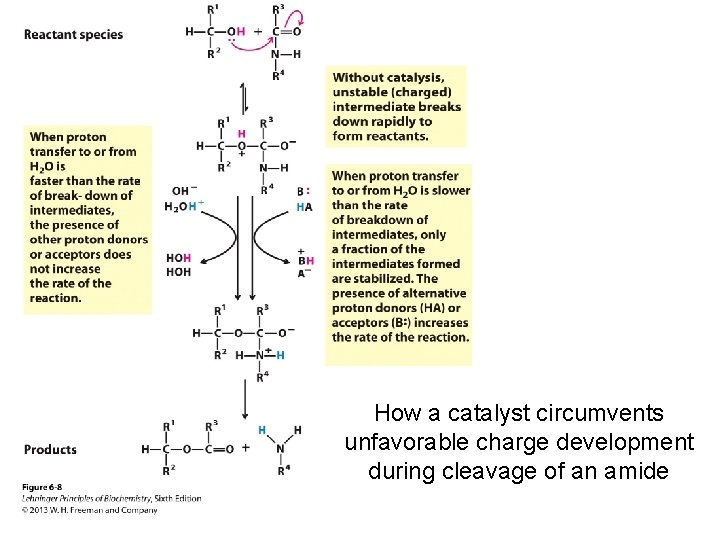
How a catalyst circumvents unfavorable charge development during cleavage of an amide

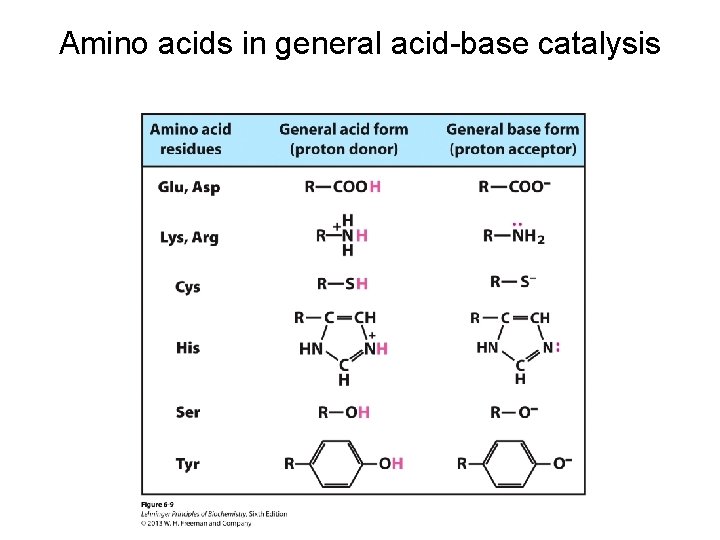
Amino acids in general acid-base catalysis

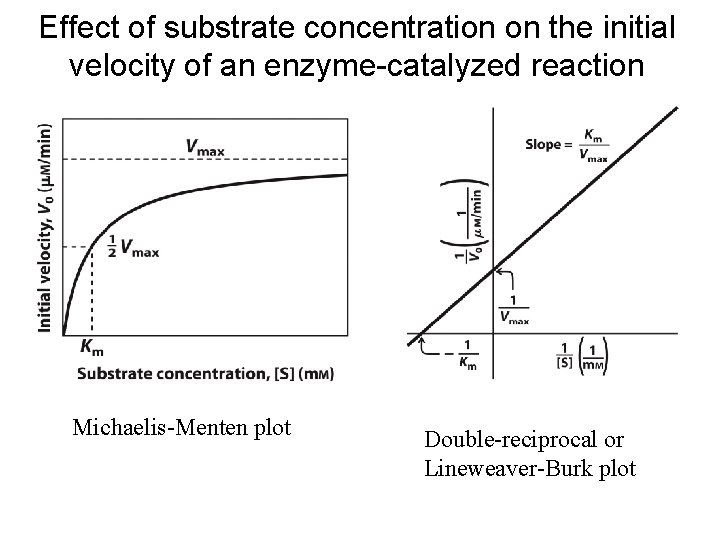
Effect of substrate concentration on the initial velocity of an enzyme-catalyzed reaction Michaelis-Menten plot Double-reciprocal or Lineweaver-Burk plot

![kcat Vmax Etotal kcat has units of reciprocal time kcat = Vmax / [E]total kcat has units of reciprocal time](https://slidetodoc.com/presentation_image_h/df4a538b8a53ffb9ce2a185a311b7090/image-15.jpg)
kcat = Vmax / [E]total kcat has units of reciprocal time

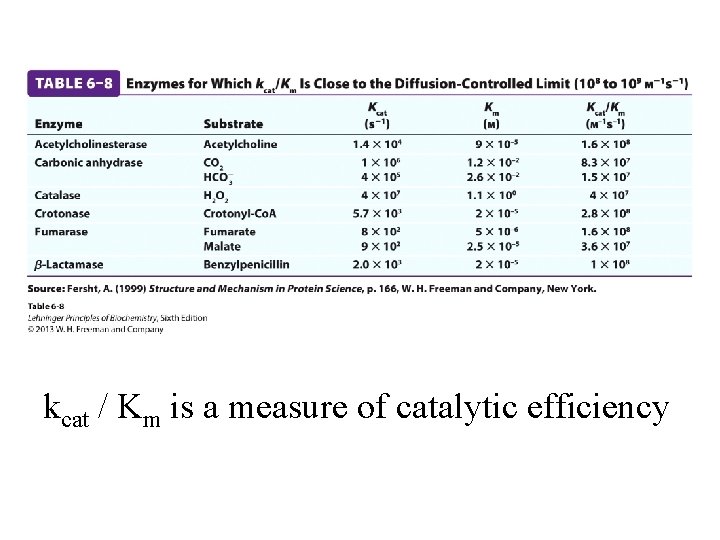
kcat / Km is a measure of catalytic efficiency

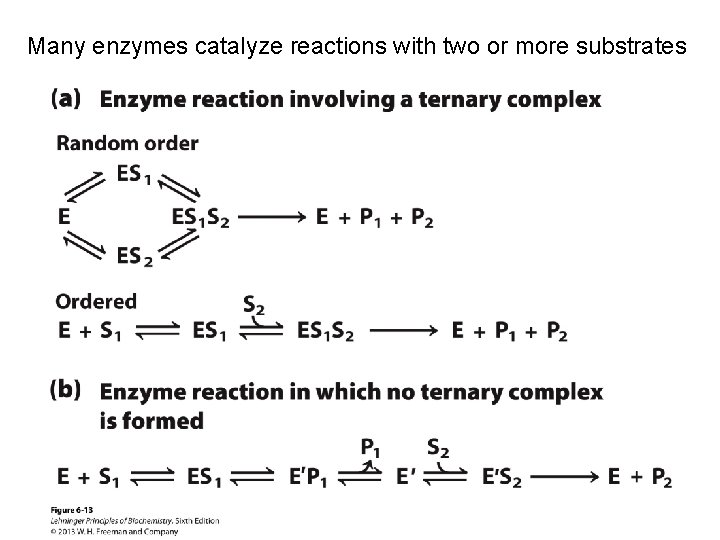
Many enzymes catalyze reactions with two or more substrates

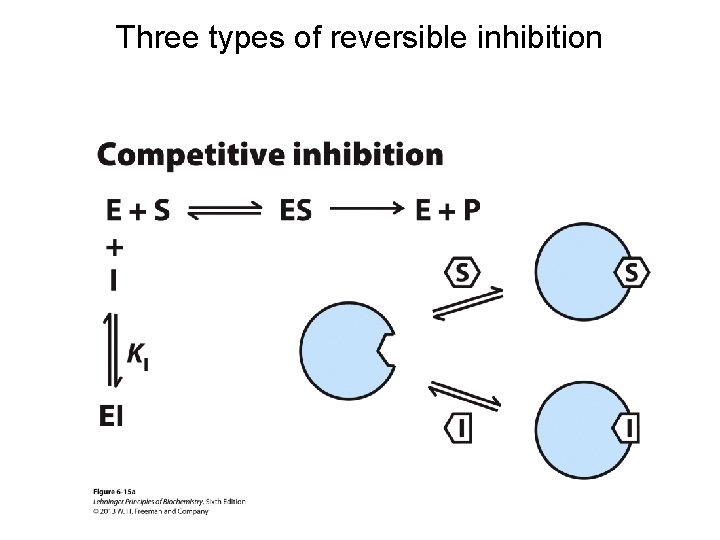
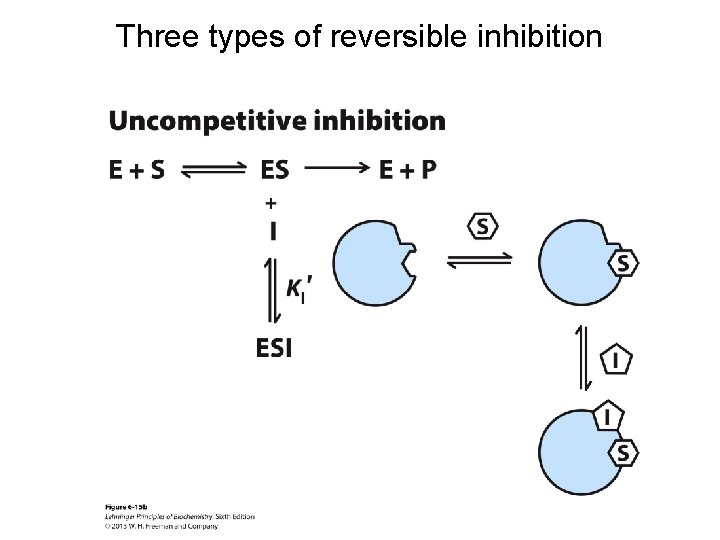
Three types of reversible inhibition

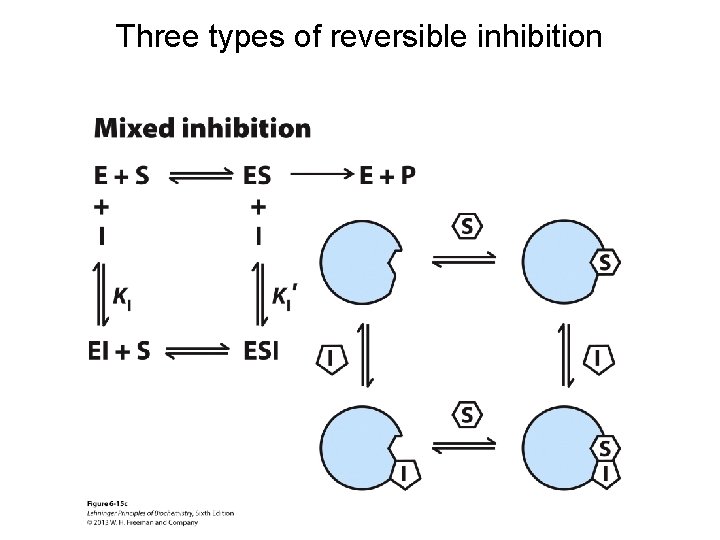
Three types of reversible inhibition

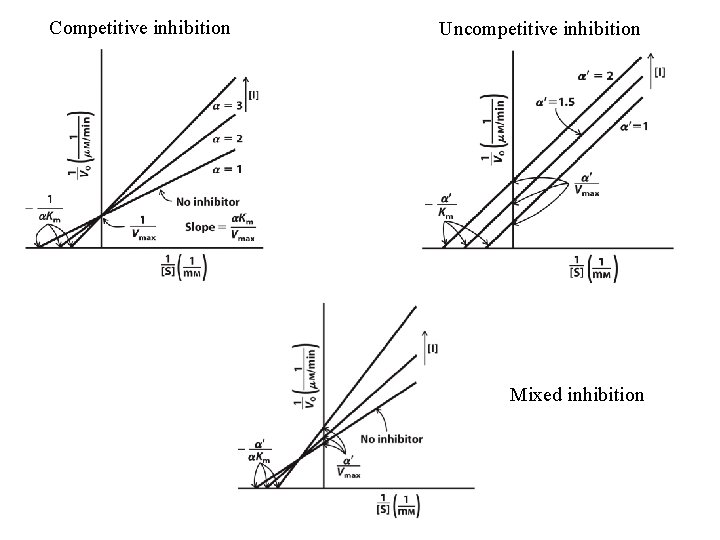
Three types of reversible inhibition

Competitive inhibition Uncompetitive inhibition Mixed inhibition

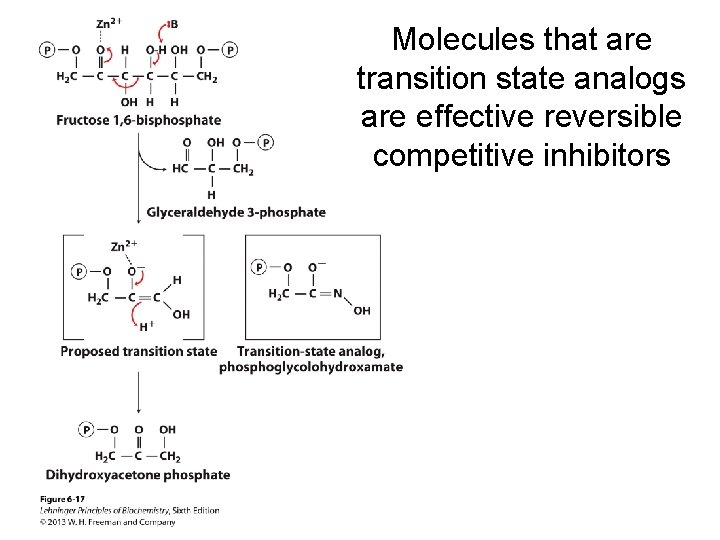
Molecules that are transition state analogs are effective reversible competitive inhibitors

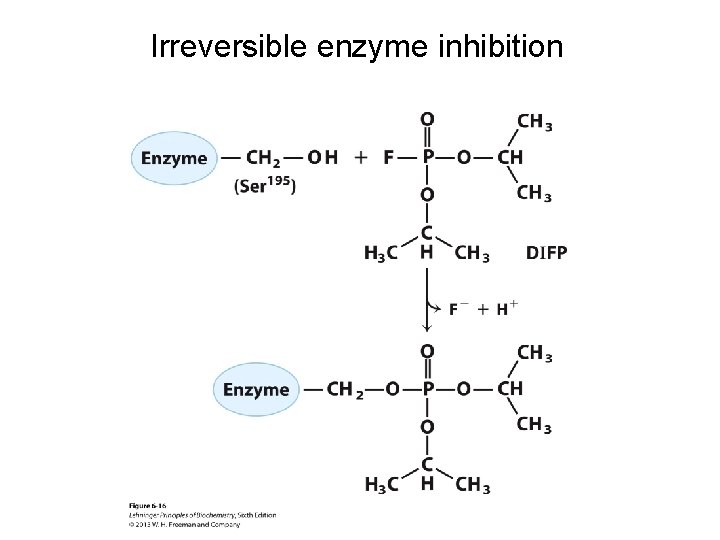
Irreversible enzyme inhibition

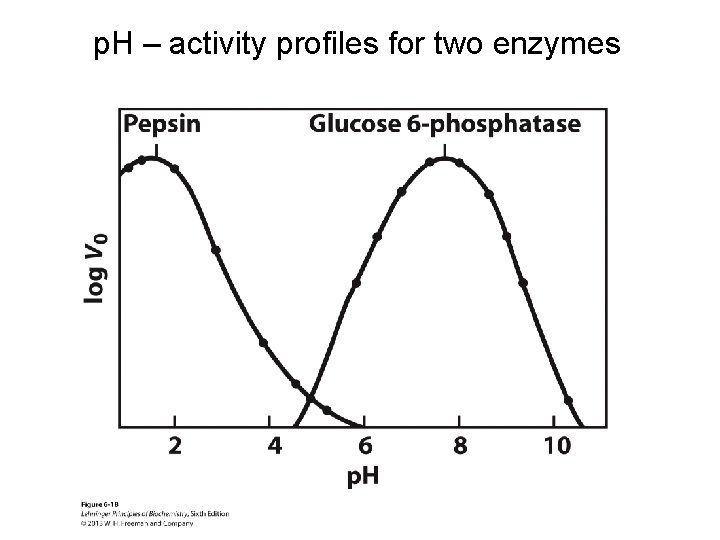
p. H – activity profiles for two enzymes

Structure of chymotrypsin, a serine protease









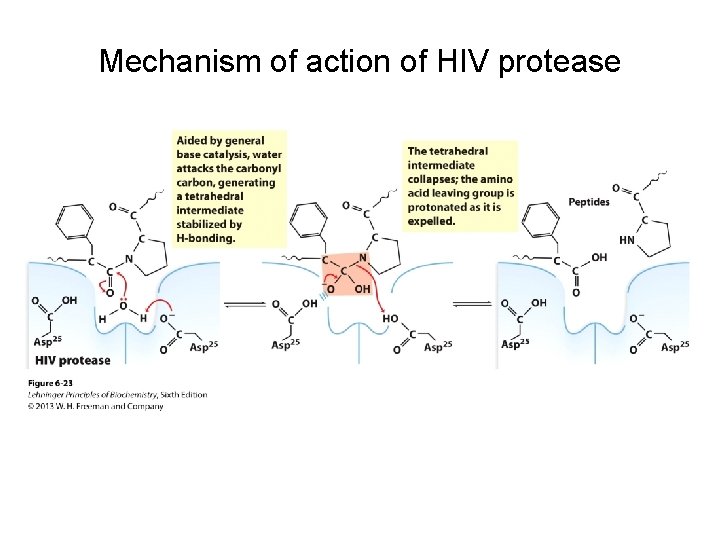
Mechanism of action of HIV protease

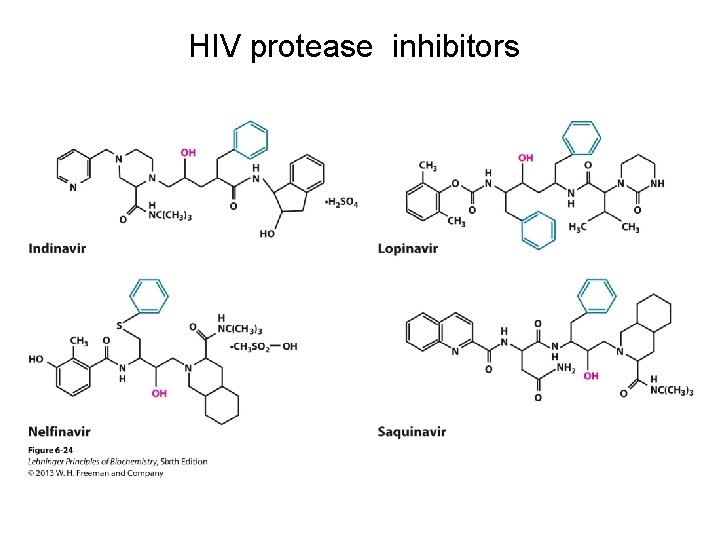
HIV protease inhibitors

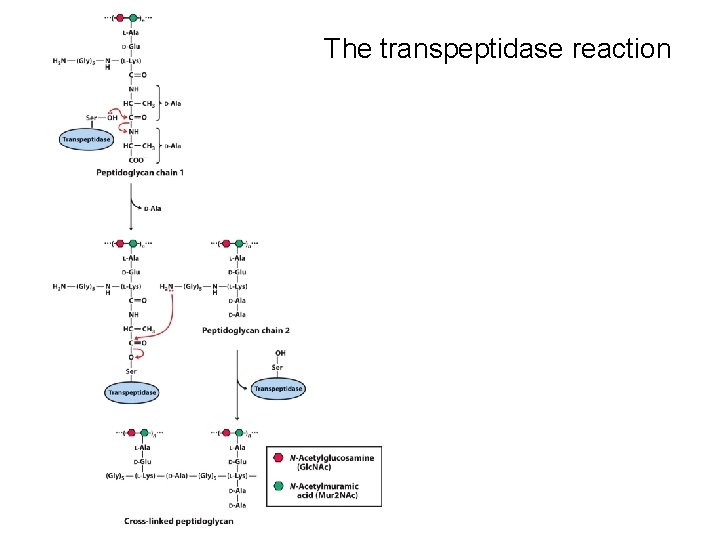
The transpeptidase reaction


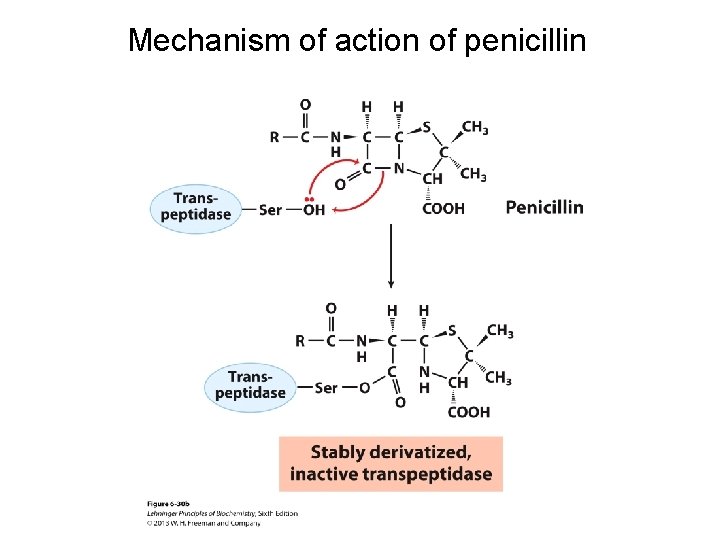
Mechanism of action of penicillin

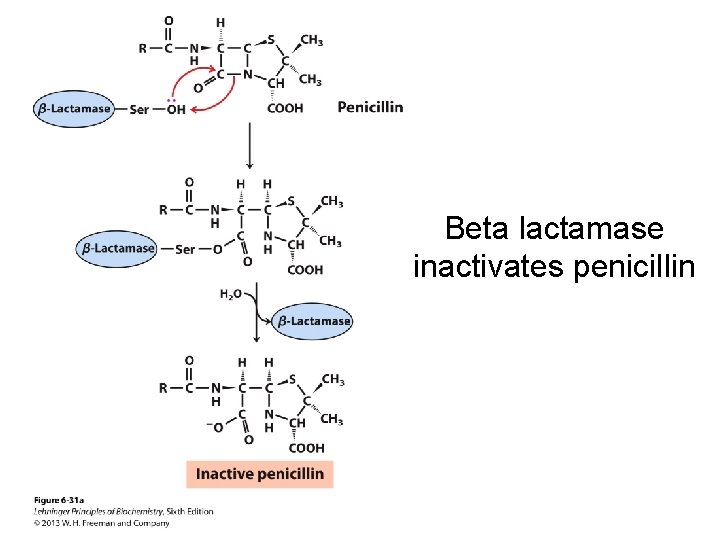
Beta lactamase inactivates penicillin

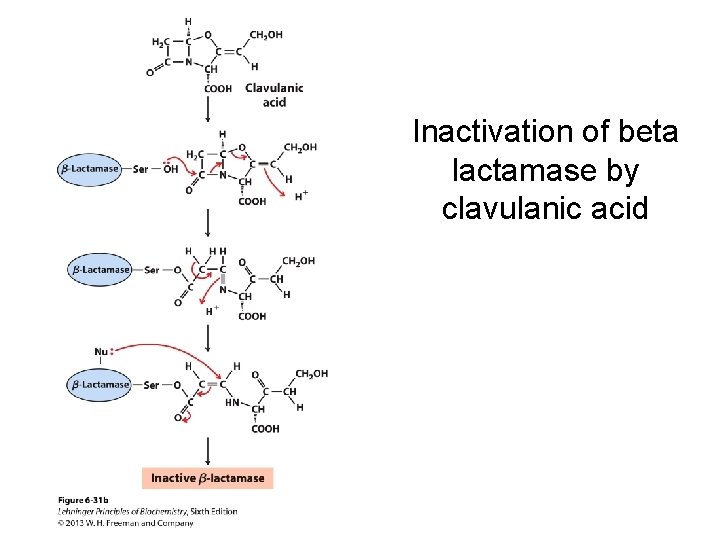
Inactivation of beta lactamase by clavulanic acid

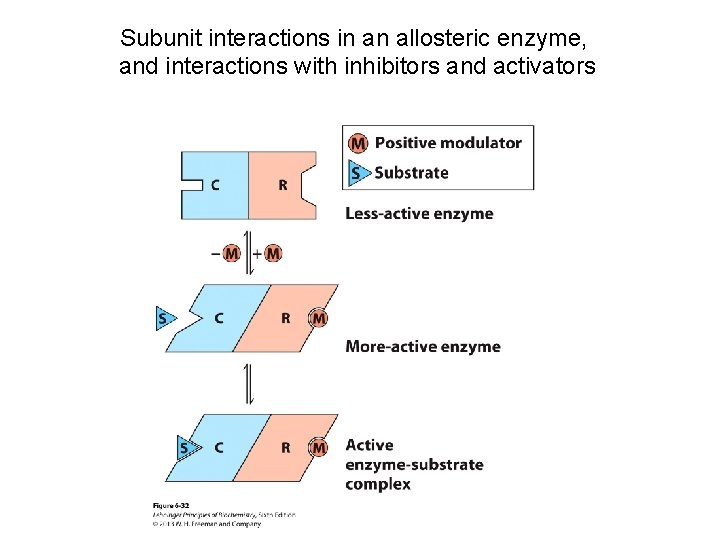
Subunit interactions in an allosteric enzyme, and interactions with inhibitors and activators

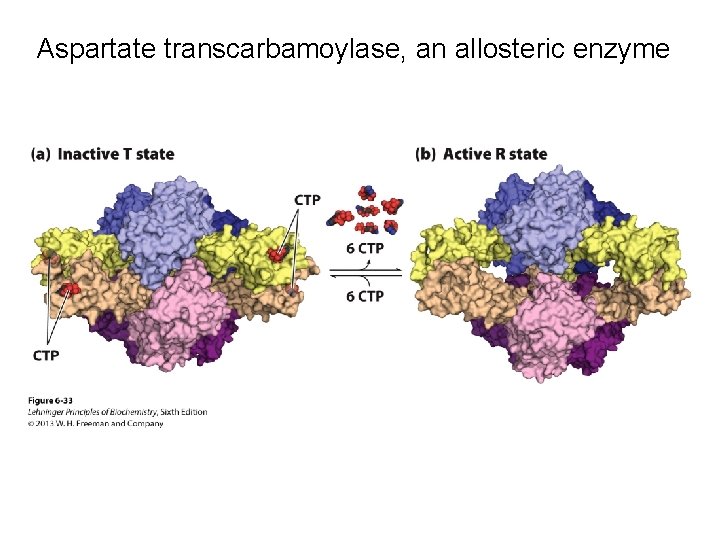
Aspartate transcarbamoylase, an allosteric enzyme

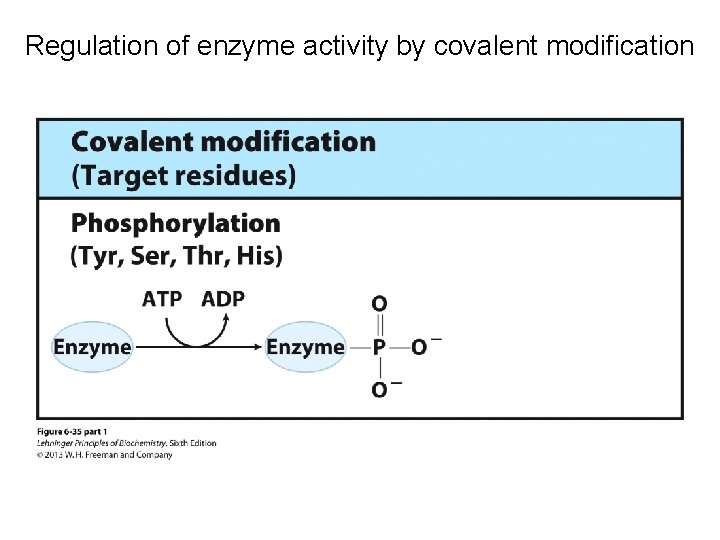
Regulation of enzyme activity by covalent modification

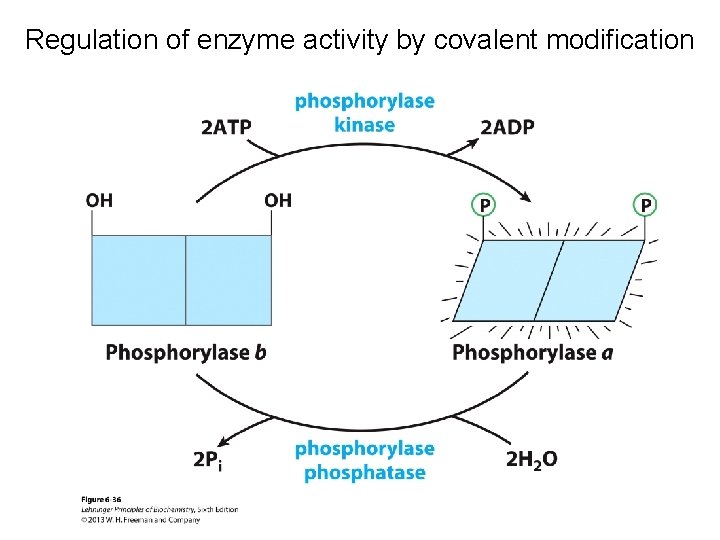
Regulation of enzyme activity by covalent modification


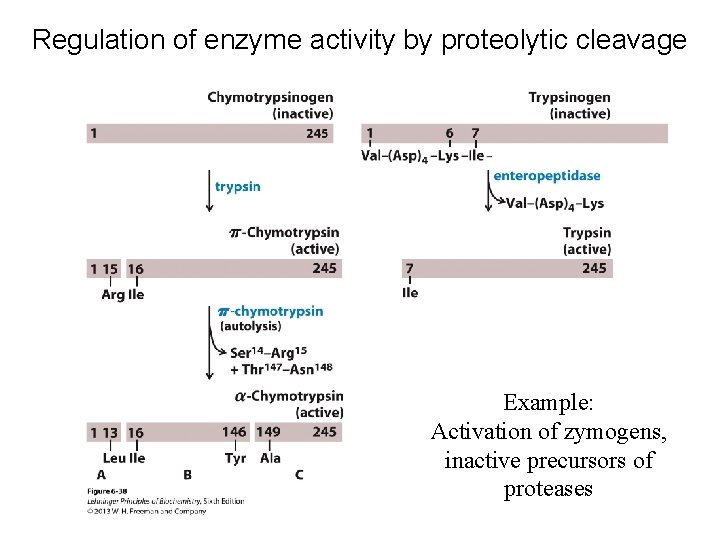
Regulation of enzyme activity by proteolytic cleavage Example: Activation of zymogens, inactive precursors of proteases
 Nelson and cox
Nelson and cox Nelson and cox
Nelson and cox Nelson and cox
Nelson and cox L
L Black and cox model
Black and cox model David michael & co
David michael & co Michael david boyle vietnam
Michael david boyle vietnam David michael hodgkinson
David michael hodgkinson Pananaliksik tungkol sa asignaturang filipino sa kolehiyo
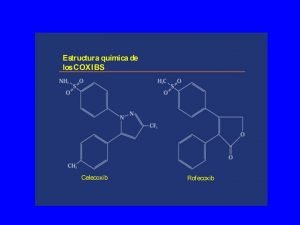
Pananaliksik tungkol sa asignaturang filipino sa kolehiyo Inhibidores selectivos de la cox-2
Inhibidores selectivos de la cox-2 Alan cox rice
Alan cox rice Ingemar j. cox
Ingemar j. cox Cox regression
Cox regression Polipi utero
Polipi utero Coxweb
Coxweb Alicia cox ph
Alicia cox ph Raymond cox qc
Raymond cox qc Jessica cox biografia
Jessica cox biografia Alan cox rice
Alan cox rice Wfo cox
Wfo cox Box cox minitab
Box cox minitab Kobe bryant relatives
Kobe bryant relatives Metoda coxa
Metoda coxa Efpractice
Efpractice Cox.net
Cox.net Prostaglandins synthesized from
Prostaglandins synthesized from Penny cox uf
Penny cox uf Cox eox tox
Cox eox tox Cox
Cox Brian cox dunedin
Brian cox dunedin An introduction to rheology
An introduction to rheology Cox emil
Cox emil Cox mill elementary school
Cox mill elementary school Tom cox intermediate
Tom cox intermediate Cox urban furniture
Cox urban furniture Tom cox intermediate
Tom cox intermediate Nele leosk
Nele leosk Rezidual doğrusallık
Rezidual doğrusallık Wash sector cox's bazar
Wash sector cox's bazar Regressiokerroin
Regressiokerroin Cox spam blocker
Cox spam blocker Cox investor relations
Cox investor relations Paul howat
Paul howat Carrie cox
Carrie cox Klovenmodel
Klovenmodel Gandhi and mandela venn diagram
Gandhi and mandela venn diagram Cliff baxter
Cliff baxter