Catalyst Deactivation Hsin Chu Professor Dept of Environmental








- Slides: 21

Catalyst Deactivation 朱信 Hsin Chu Professor Dept. of Environmental Eng. National Cheng Kung University 1

1. Introduction Deactivation a. high temperature exposure: automobile catalytic converter, close to 1000℃ b. poisoning: exhaust or process contaminants adsorbing onto or blocking active sites c. attrition and erosion of the washcoat from the support l Model Reaction A convenient tool for studying deactivation and regeneration l 2

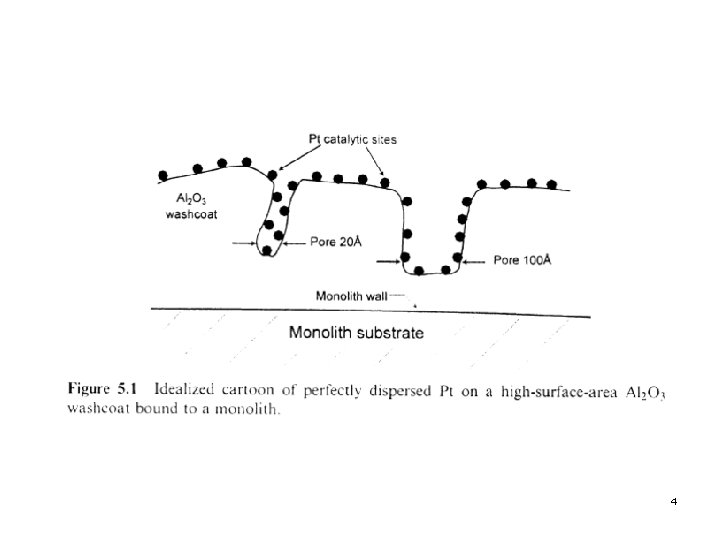
2. Thermally Induced Deactivation l. A perfectly dispersed (100% dispersion) catalyst is one in which every atom (or molecule) of active component is available to the reactants. This is shown is Fig. 5. 1 (next slide). 3

4

Some catalysts are made in this highly active state but are highly unstable, and thermal effects cause crystal growth, resulting in a loss of catalytic surface area. l Additionally, the carrier with a large internal surface network of pores tends to undergo sintering with a consequent loss in internal surface area. l Besides, reactions of the catalytically active species with the carrier, resulting in the formation of a less catalytically active species. l 5

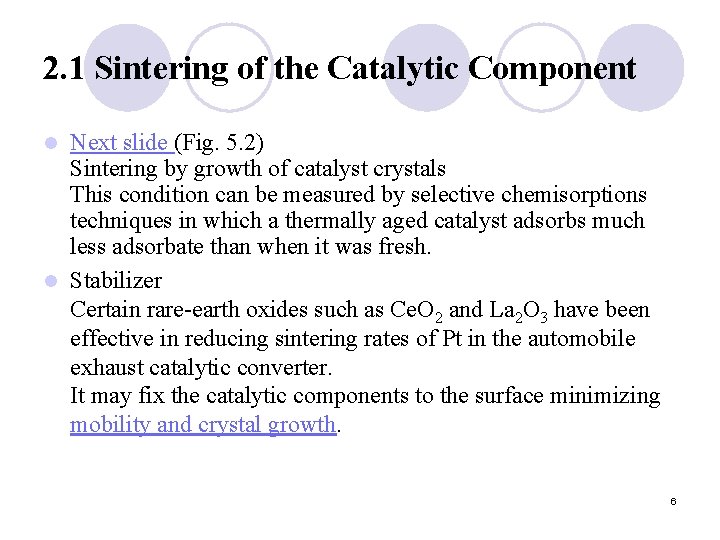
2. 1 Sintering of the Catalytic Component Next slide (Fig. 5. 2) Sintering by growth of catalyst crystals This condition can be measured by selective chemisorptions techniques in which a thermally aged catalyst adsorbs much less adsorbate than when it was fresh. l Stabilizer Certain rare-earth oxides such as Ce. O 2 and La 2 O 3 have been effective in reducing sintering rates of Pt in the automobile exhaust catalytic converter. It may fix the catalytic components to the surface minimizing mobility and crystal growth. l 6

7

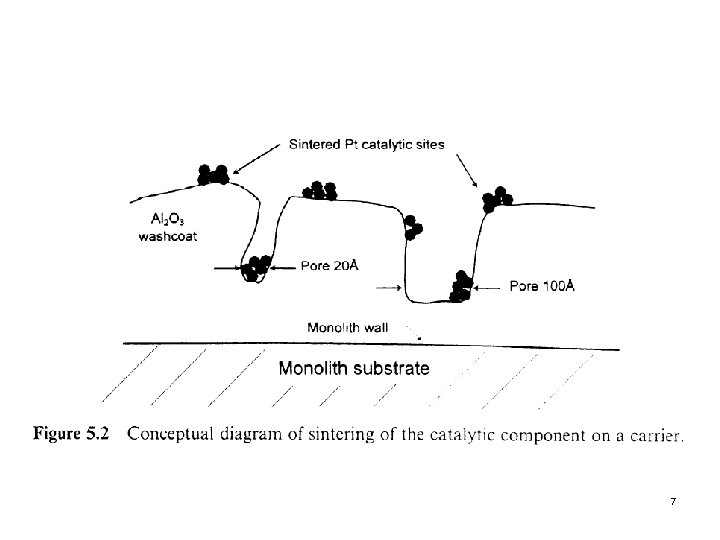
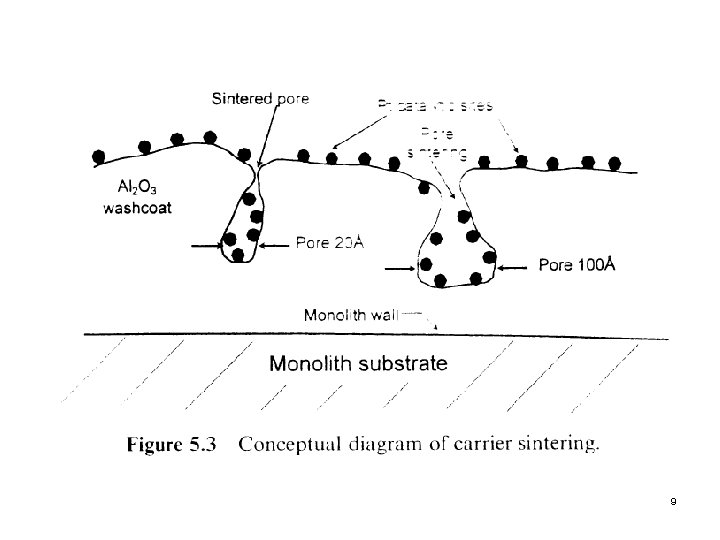
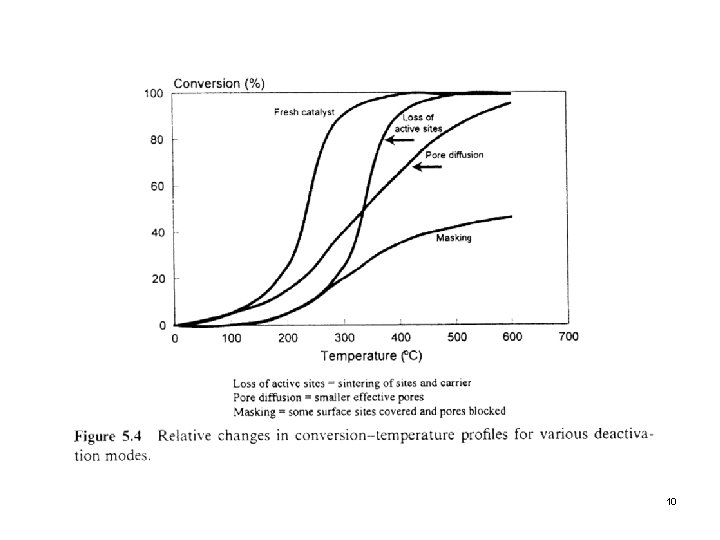
2. 2 Carrier Sintering Within a given crystal structure, such as γ-Al 2 O 3, the loss of surface area is associated with loss of H 2 O and a gradual loss of the internal pore structure network, as shown in the next slide (Fig. 5. 3) l The presence of these phenomena is determined by a progressive decrease in the activation energy of the reaction. l Second slide (Fig. 5. 4) Conversion profiles for various deactivation modes l 8

9

10

Second mechanism for carrier change in crystal structure γ-Al 2 O 3 → α-Al 2 O 3 150 m 2/g < 5 m 2/g Anatase Ti. O 2 Rutile Ti. O 2 60 m 2/g < 10 m 2/g l Stabilizer Ba. O, La 2 O 3, Si. O 2, or Zr. O 2 can retard the rate of sintering in certain carriers. They are believed to form solid solutions with the carrier surface, decreasing their surface reactivity, which leads to sintering. l 11

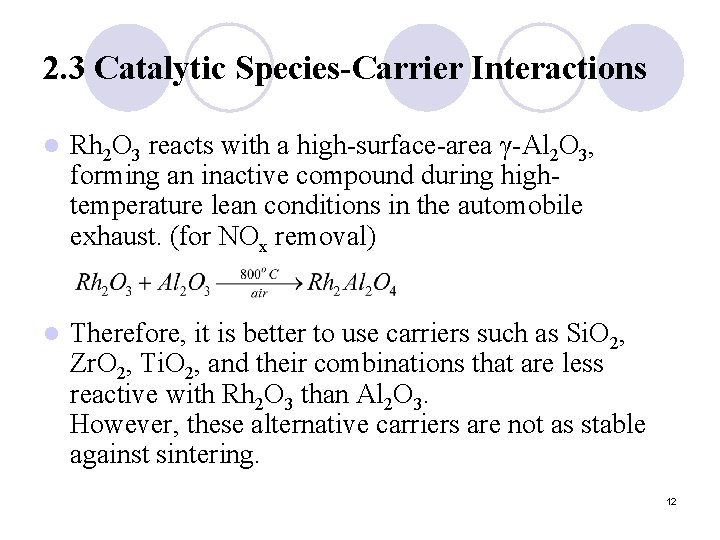
2. 3 Catalytic Species-Carrier Interactions l Rh 2 O 3 reacts with a high-surface-area γ-Al 2 O 3, forming an inactive compound during hightemperature lean conditions in the automobile exhaust. (for NOx removal) l Therefore, it is better to use carriers such as Si. O 2, Zr. O 2, Ti. O 2, and their combinations that are less reactive with Rh 2 O 3 than Al 2 O 3. However, these alternative carriers are not as stable against sintering. 12

3. Poisoning l Selective poisoning A chemical directly reacts with the active site or the carrier, rendering it less or completely inactive. l Nonselective poisoning Deposition of fouling agents onto or into the catalyst carrier, masking sites and pores, resulting in a loss in performance. 13

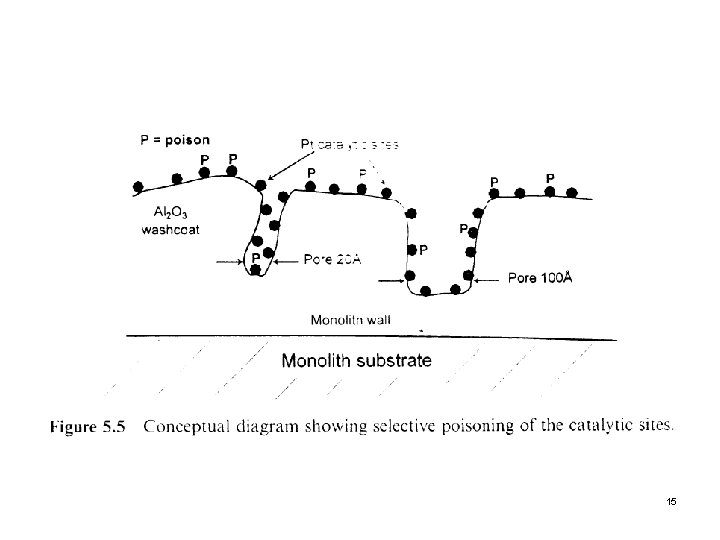
3. 1 Selective Poisoning Next slide (Fig. 5. 5) A poison directly reacts with an active site l Permanent deactivation Pb, Hg, and Cd react directly with Pt, forming a catalytically inactive alloy. l Reversible deactivation SO 2 merely adsorbs onto a metal site (i. e. , Pd). Heat treatment, washing, or simply removing the poison from the process stream, often desorbs the poison from the catalytic site and restoring its catalytic activity. l 14

15

When active sites are directly poisoned, there is a shift to high temperature but with no change in the slope of conversion profile since the remaining sites can function as before with no change in activation energy. l When the carrier reacts with a constituent in the gas stream to form a new compounds, as in the case of Al 2(SO 4)3, pores are generally partially blocked, resulting in increased diffusion resistance. This will cause a decrease in the activation energy. l 16

SO 2 chemisorbs onto Pd/γ-Al 2 O 3, causing deactivation for methane oxidation. Some of the adsorbed SO 2 is converted to SO 3, which spills over, forming Al 2(SO 4)3. Using nonsulfating carriers such as Zr. O 2 or Si. O 2 leads to a faster rate of deactivation since no reservoir is available for spillover. l With the Pt/γ-Al 2 O 3 catalyst the SO 2 is readily converted to SO 3, which rapidly desorbs and reacts with the Al 2 O 3, forming Al 2(SO 4)3, which slowly causes pore plugging. By using nonsulfating carriers, the Pt catalyst can be made resistant to deactivation. l 17

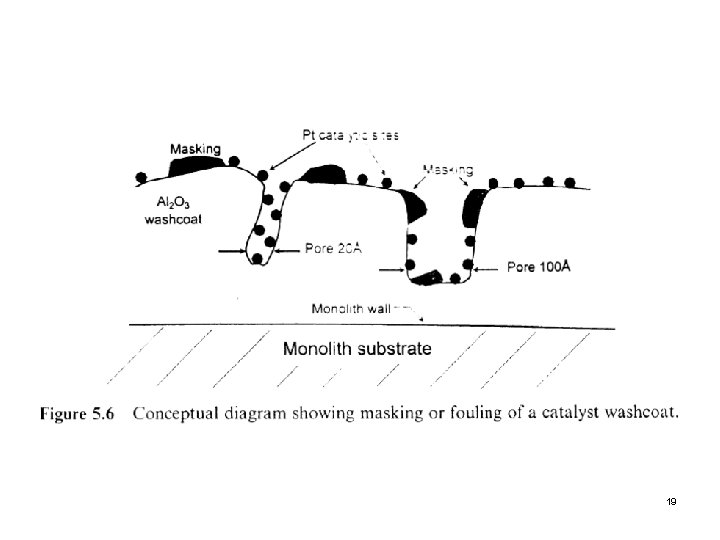
3. 2 Nonselective Poisoning Aerosol or high-molecular-weight material from upstream equipment physically deposit onto the surface of the washcoat to cause deactivation is referred to as “fouling” or “masking”. l Reactor-scale metals (Fe, Ni, Cr, etc. ) resulting from corrosion, silica/alumina-containing dusts, phosphorous from lubricating oils, and similar compounds are good examples. l Next slide (Fig. 5. 6) Masking or fouling of a catalyst washcoat l Second slide (Fig. 5. 7) SEM of fresh and aged Pt/Al 2 O 3 surfaces l 18

19

20

4. Washcoat Loss l Attrition or Erosion Irreversible deactivation a. high linear velocities of gas flow b. thermal expansion differences between the washcoat and the monolith, especially metal substrates 21
 Arus pu chu chu
Arus pu chu chu Trò chơi chữ cái u ư chủ đề nghề nghiệp
Trò chơi chữ cái u ư chủ đề nghề nghiệp Muốn tính chu vi hình chữ nhật
Muốn tính chu vi hình chữ nhật Muốn tính chu vi hình chữ nhật
Muốn tính chu vi hình chữ nhật Word formation
Word formation Hsin chong construction group ltd
Hsin chong construction group ltd Promotion from assistant to associate professor
Promotion from assistant to associate professor Mh 605
Mh 605 Gome dept
Gome dept Ee dept iitb
Ee dept iitb Inspectors in worcester
Inspectors in worcester Tabella nmr
Tabella nmr Hoe dept
Hoe dept Employment first ohio
Employment first ohio La geaux biz
La geaux biz Florida dept of agriculture and consumer services
Florida dept of agriculture and consumer services Dept nmr spectroscopy
Dept nmr spectroscopy Central islip fire department
Central islip fire department Gome dept
Gome dept Dept ind onegov
Dept ind onegov Dept. name of organization
Dept. name of organization Pt dept logistik
Pt dept logistik