Carl Deirmengian MD Scientific Founder and Chief Scientific












- Slides: 21

Carl Deirmengian, MD Scientific Founder and Chief Scientific Officer January 18, 2011 - PCCI OVERVIEW

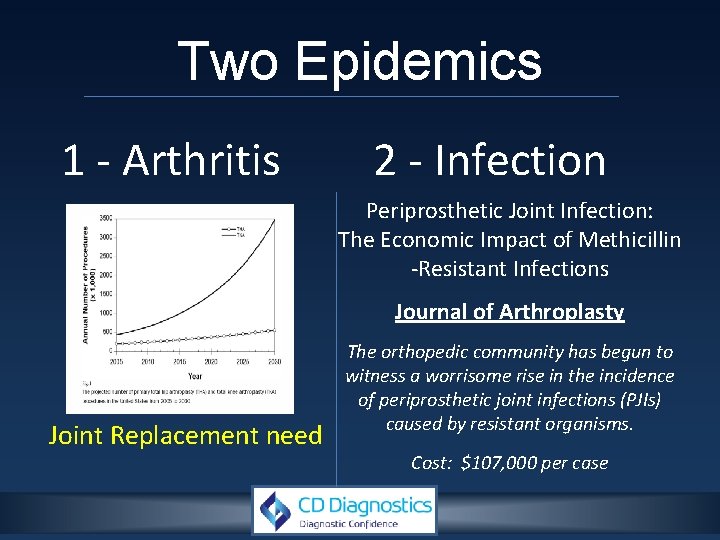
Two Epidemics 1 - Arthritis 2 - Infection Periprosthetic Joint Infection: The Economic Impact of Methicillin -Resistant Infections Journal of Arthroplasty Joint Replacement need The orthopedic community has begun to witness a worrisome rise in the incidence of periprosthetic joint infections (PJIs) caused by resistant organisms. Cost: $107, 000 per case

The Problem Infections are not obvious Antibiotics alone do not cure deep joint infections The U. S. standard of care involves a two-stage surgical procedure over three months

Standard Diagnostics • Blood tests – CRP, ESR • Intuitively suboptimal • Joint Tests – WBC count, Cultures • Subjective, inconsistent • Radiographic – MRI, Bone scan, PET scan THE SURGEON IS LEFT WITHOUT CONFIDENCE

r Cygnasure Addresses an unmet clinical market need • Tests the local joint fluid • Objective analytic laboratory test • Rapid point-of-care • Improved accuracy

Technology Based on antibody technology (ELISA) • > 25 year history • No technological barriers • Multiple platforms • Low cost of development

3 Clinical Studies #1 – Coventry Award Publication 12 knees – defined genetic signature for infection #2 – Confirmation study 16 knees – confirmed genetic signature #3 – Clinical Orthopaedics 51 knees – ELISA benchmark study

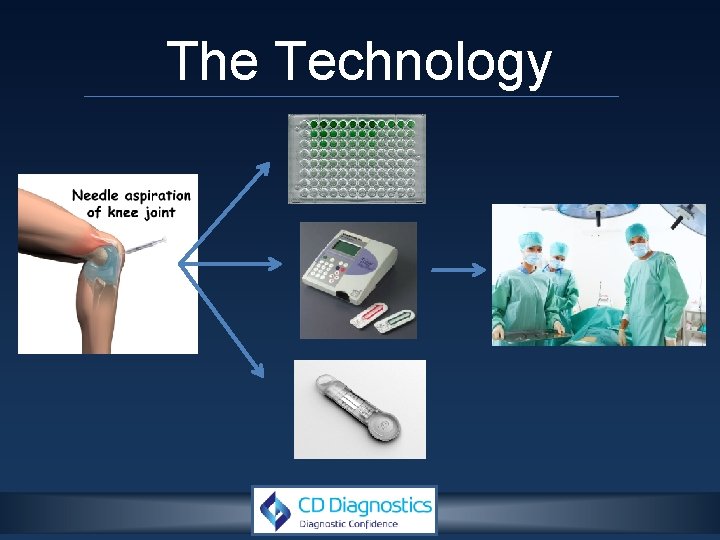
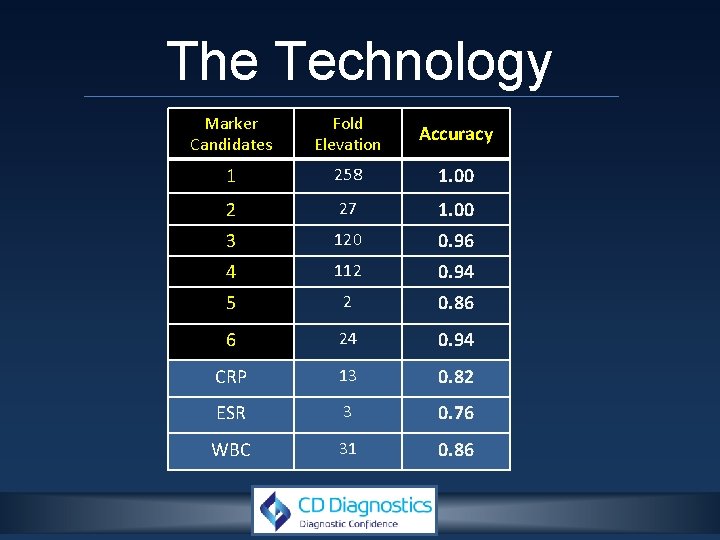
The Technology

The Technology Marker Candidates Fold Elevation Accuracy 1 258 1. 00 2 27 1. 00 3 120 0. 96 4 112 0. 94 5 2 0. 86 6 24 0. 94 CRP 13 0. 82 ESR 3 0. 76 WBC 31 0. 86

Clinical Awards • 2006 American Academy of Orthopaedic Surgery Poster Award • 2005 The Knee Society, Mark Coventry National Award (1 st) • 2005 OREF/Zimmer Career Development Award • 2004 OREF Resident Research Grant/Award • 2004 The Smith & Nephew National Research Award (1 st) • 2004 National Arthroscopy Association Resident Award (1 st)

Intellectual Property USPTO # 7598080 October 6, 2009 Diagnostic Assay for the Source of Inflammation

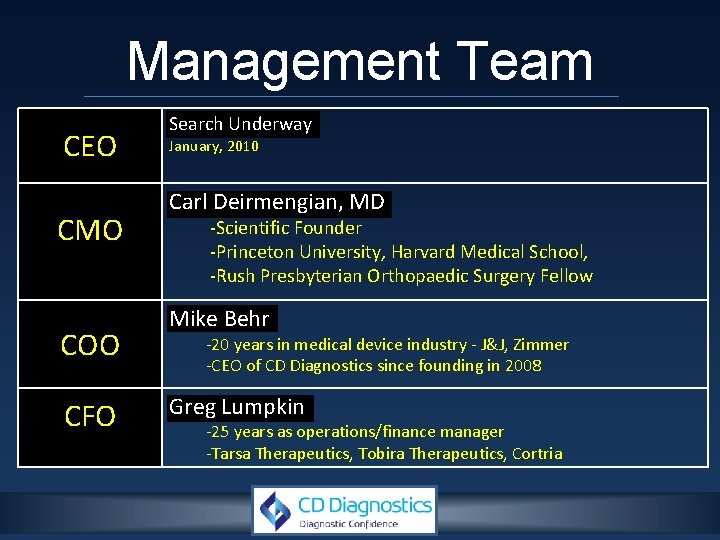
Management Team CEO CMO COO CFO Search Underway January, 2010 Carl Deirmengian, MD -Scientific Founder -Princeton University, Harvard Medical School, -Rush Presbyterian Orthopaedic Surgery Fellow Mike Behr -20 years in medical device industry - J&J, Zimmer -CEO of CD Diagnostics since founding in 2008 Greg Lumpkin -25 years as operations/finance manager -Tarsa Therapeutics, Tobira Therapeutics, Cortria

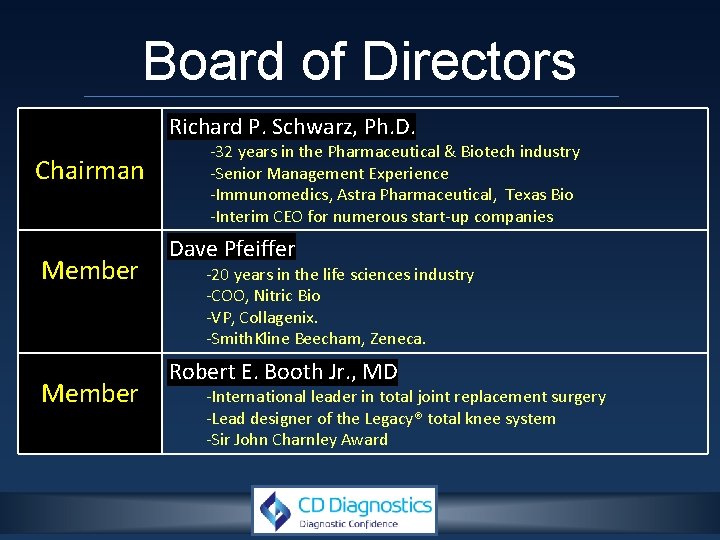
Board of Directors Richard P. Schwarz, Ph. D. Chairman Member -32 years in the Pharmaceutical & Biotech industry -Senior Management Experience -Immunomedics, Astra Pharmaceutical, Texas Bio -Interim CEO for numerous start-up companies Dave Pfeiffer -20 years in the life sciences industry -COO, Nitric Bio -VP, Collagenix. -Smith. Kline Beecham, Zeneca. Robert E. Booth Jr. , MD -International leader in total joint replacement surgery -Lead designer of the Legacy® total knee system -Sir John Charnley Award

Scientific Advisory Board Javad Parvizi, MD – International Leader in Orthopaedic Infection BOD – Orthopaedic Research Society and Musculoskeletal Infections Jess Lonner, MD – International Leader in Orthopaedic Infection BOD – Orthopaedic Research Society Craig Della Valle, MD – International Leader in Orthopaedic Infection Chairman AAOS Infection Guidelines Committee Alan Wu, Ph. D. – Chief Clinical Chemistry, UCSF >20 awards in the field of molecular diagnostics, protein biomarkers Sam Niedbala, Ph. D. – Founder and Chief Scientific Officer, Orasure

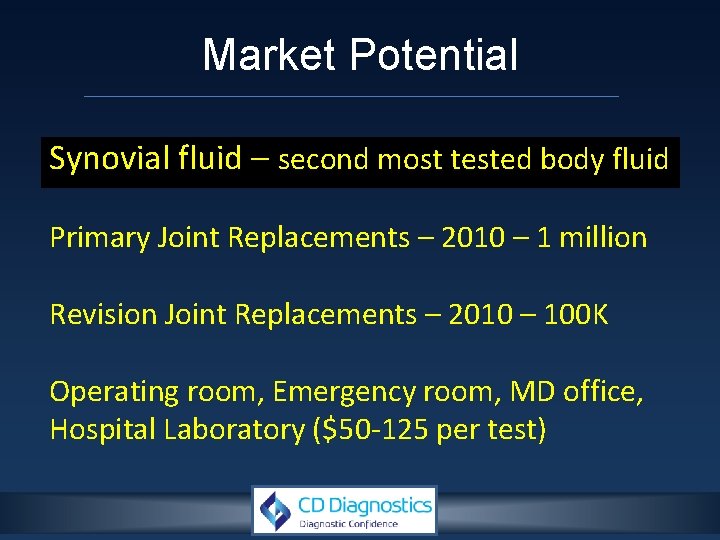
Market Potential Synovial fluid – second most tested body fluid Primary Joint Replacements – 2010 – 1 million Revision Joint Replacements – 2010 – 100 K Operating room, Emergency room, MD office, Hospital Laboratory ($50 -125 per test)

Market Potential Synovial fluid – second most tested body fluid Rheumatoid arthritis, Lupus, Lyme disease, Gout, pseudoseptic infection, pseudogout

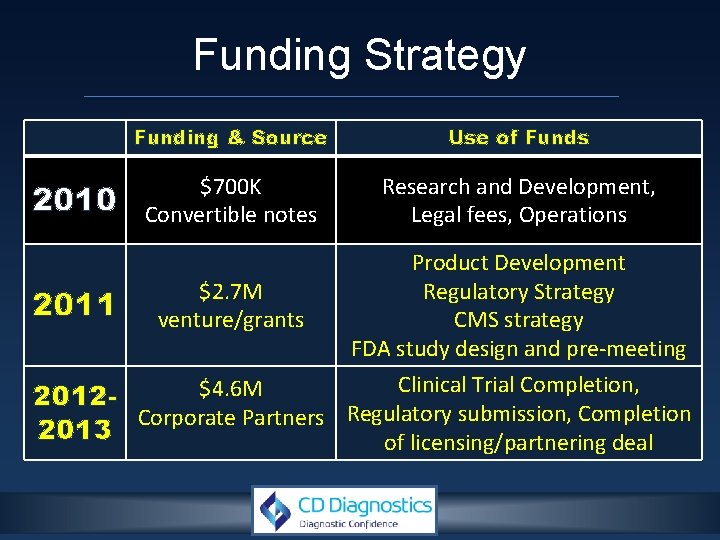
Funding Strategy 2010 2011 Funding & Source Use of Funds $700 K Convertible notes Research and Development, Legal fees, Operations $2. 7 M venture/grants Product Development Regulatory Strategy CMS strategy FDA study design and pre-meeting Clinical Trial Completion, $4. 6 M 2012 Corporate Partners Regulatory submission, Completion 2013 of licensing/partnering deal

Successful Exit CD Diagnostics – The Synovial Fluid Company - Intellectual property portfolio Inflection point – FDA approval 1. 2. 3. Licensing to a corporate partner - large diagnostics Strategic buyer - mid-range diagnostics, orthopaedics IPO

Next Step • Identify and hire CEO • Create and Execute - Product development plan - Clinical trial plan - Regulatory strategy • Close additional financings


Questions 1. CD Diagnostics has a solid technology with a strong patent that might increase in scope with a patent continuance that looks promising. Is it a better idea for CD Diagnostics to put all of it’s energy into developing one product and selling the company, with it’s technology, to a potential buyer or would it be better to develop an extended line of applications for this technology and then approach a potential buyer/partner? 2. CD Diagnostics has been approached by a couple of large companies that might be interested in a strategic partnership. Is a strategic partnership a good idea at this point for CD Diagnostics? What models for a strategic partnership would make sense? 3. CD Diagnostics is looking at models for pricing of Cygnasure®. What models exist to determine an appropriate price for a product like Cygnasure®? How should we begin to determine a price for Cygnasure®.
 Carl
Carl Chapter 9 lesson 3 commander in chief and chief diplomat
Chapter 9 lesson 3 commander in chief and chief diplomat Who the father of scientific management
Who the father of scientific management Scientific inquiry vs scientific method
Scientific inquiry vs scientific method How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Daoism founder
Daoism founder Walt disney world founders
Walt disney world founders The founder of utilitarianism
The founder of utilitarianism Tracy david swena
Tracy david swena Total quality management founder
Total quality management founder Total quality management founder
Total quality management founder Where is plymouth
Where is plymouth The founder of buddhism
The founder of buddhism Basics of sikhism
Basics of sikhism Judaism founder
Judaism founder Evolutionary drift
Evolutionary drift Founder of utilitarianism
Founder of utilitarianism Devshirme system
Devshirme system Kfc company
Kfc company History of samaritan's purse
History of samaritan's purse Where did judo originate
Where did judo originate Hinduism founder/origins
Hinduism founder/origins









































