Calculations of phase diagrams using ThermoCalc software package

- Slides: 15

Calculations of phase diagrams using Thermo-Calc software package Content Equilibrium calculation using the Gibbs energy minimisation 1. The Gibbs energy for a system 2. The Gibbs energy for a phase Unary system: Sn (calculation of melting temperature, plotting thermodynamic functions) Phase diagram for the Sn-Bi system (Temperature - Composition) Calculation of invariant reaction (T, phase compositions, enthalpy) Calculation of thermodynamic properties of liquid phase Calculation of phase fraction diagram for Bi concentration 5, 25 and 43 mol. % Scheil solidification simulation for Sn-Bi alloys Calculation of phase diagram for Fe-C system 1. Stable diagram 2. Metastable diagram

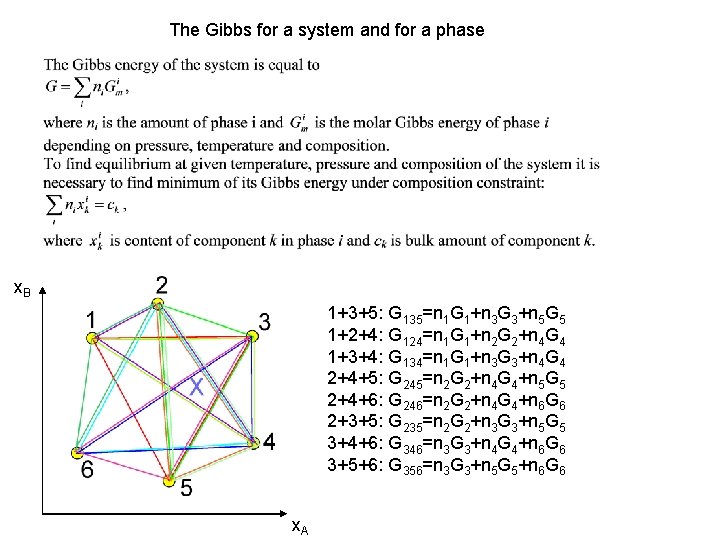
The Gibbs for a system and for a phase x. B 1+3+5: G 135=n 1 G 1+n 3 G 3+n 5 G 5 1+2+4: G 124=n 1 G 1+n 2 G 2+n 4 G 4 1+3+4: G 134=n 1 G 1+n 3 G 3+n 4 G 4 2+4+5: G 245=n 2 G 2+n 4 G 4+n 5 G 5 2+4+6: G 246=n 2 G 2+n 4 G 4+n 6 G 6 2+3+5: G 235=n 2 G 2+n 3 G 3+n 5 G 5 3+4+6: G 346=n 3 G 3+n 4 G 4+n 6 G 6 3+5+6: G 356=n 3 G 3+n 5 G 5+n 6 G 6 x. A

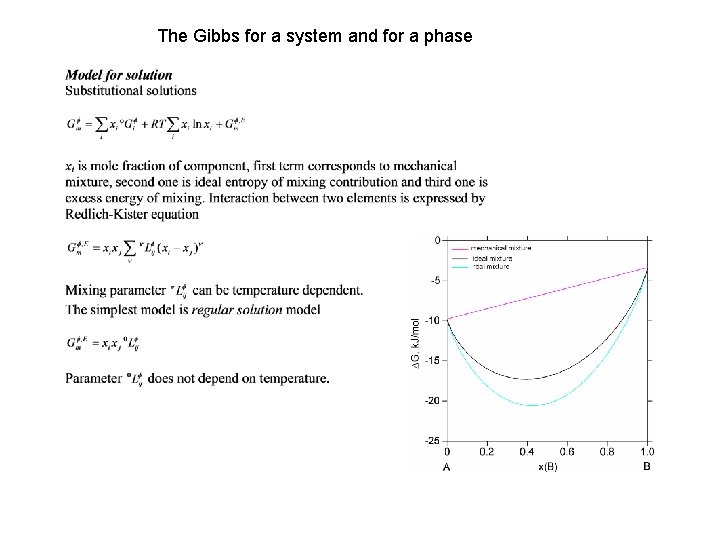
The Gibbs for a system and for a phase

The Gibbs for a system and for a phase

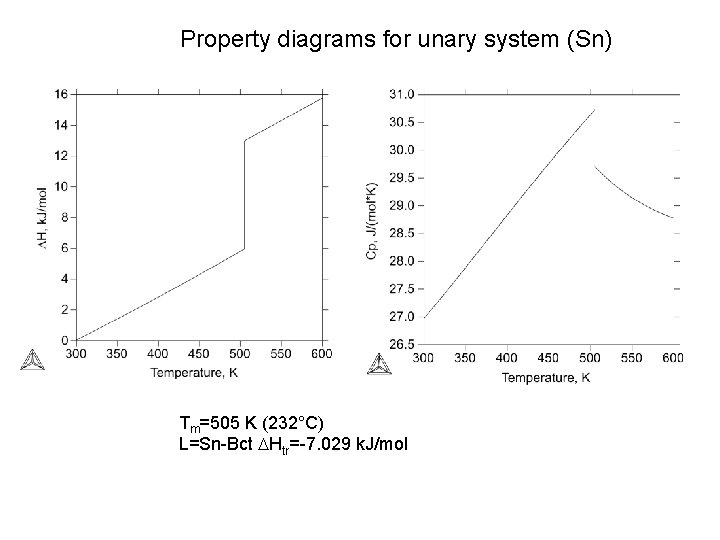
Property diagrams for unary system (Sn) Tm=505 K (232°C) L=Sn-Bct DHtr=-7. 029 k. J/mol

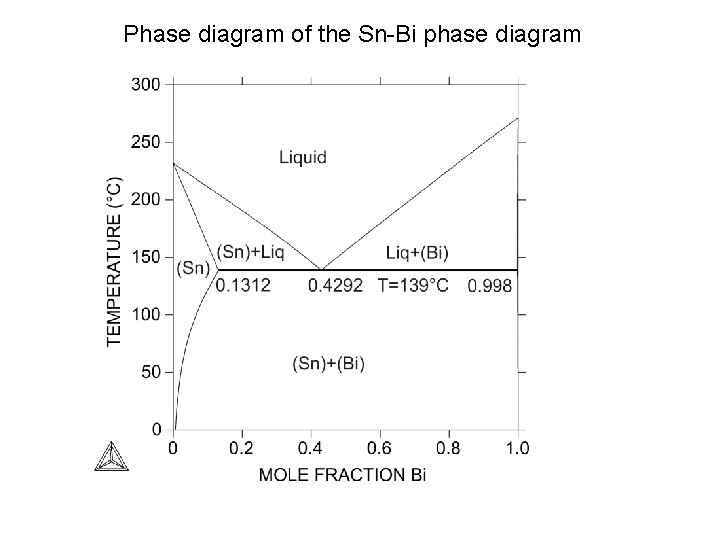
Phase diagram of the Sn-Bi phase diagram

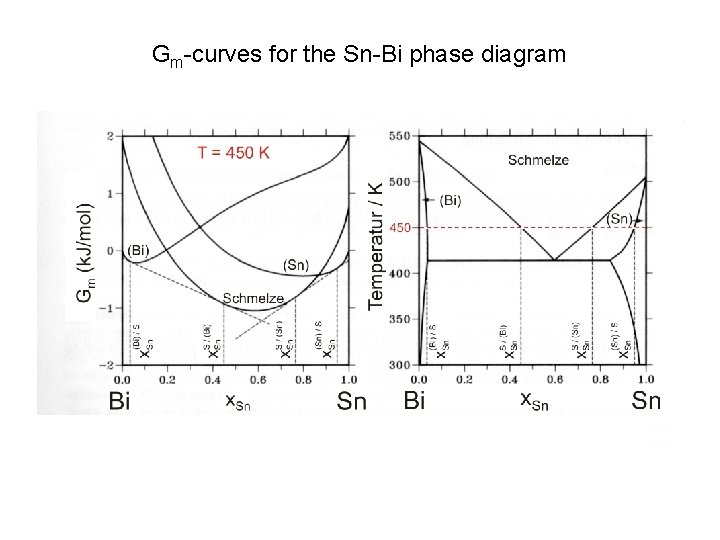
Gm-curves for the Sn-Bi phase diagram

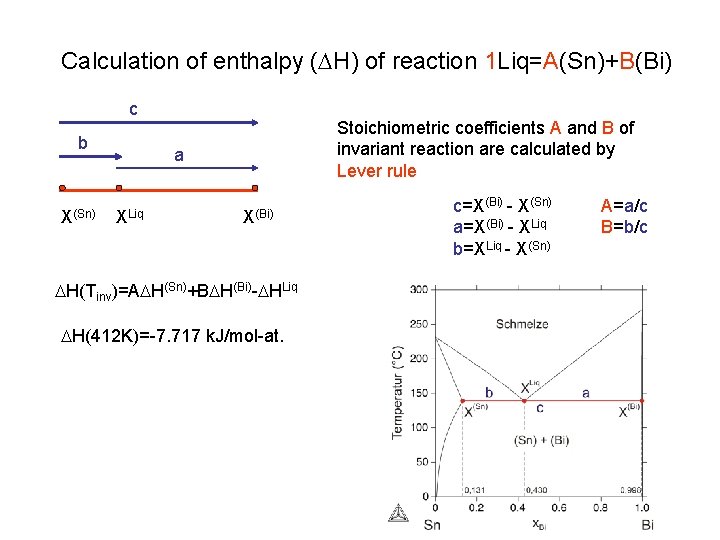
Calculation of enthalpy (DH) of reaction 1 Liq=A(Sn)+B(Bi) c b X(Sn) Stoichiometric coefficients A and B of invariant reaction are calculated by Lever rule a XLiq X(Bi) DH(Tinv)=ADH(Sn)+BDH(Bi)-DHLiq DH(412 K)=-7. 717 k. J/mol-at. c=X(Bi) - X(Sn) a=X(Bi) - XLiq b=XLiq - X(Sn) A=a/c B=b/c

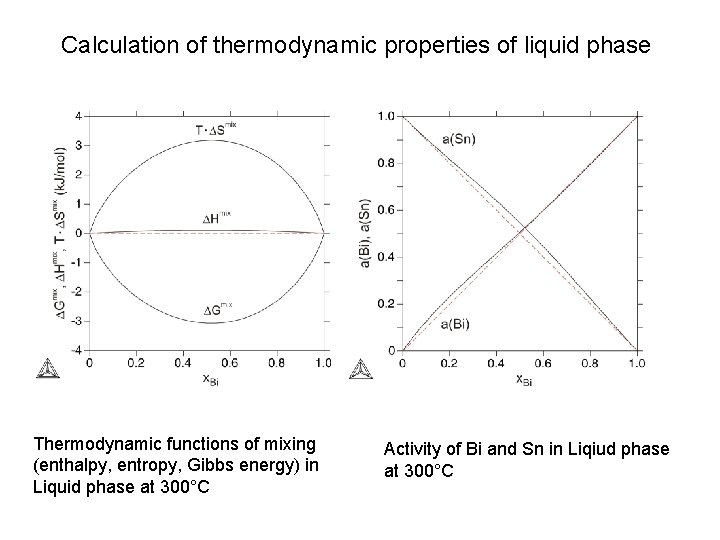
Calculation of thermodynamic properties of liquid phase Thermodynamic functions of mixing (enthalpy, entropy, Gibbs energy) in Liquid phase at 300°C Activity of Bi and Sn in Liqiud phase at 300°C

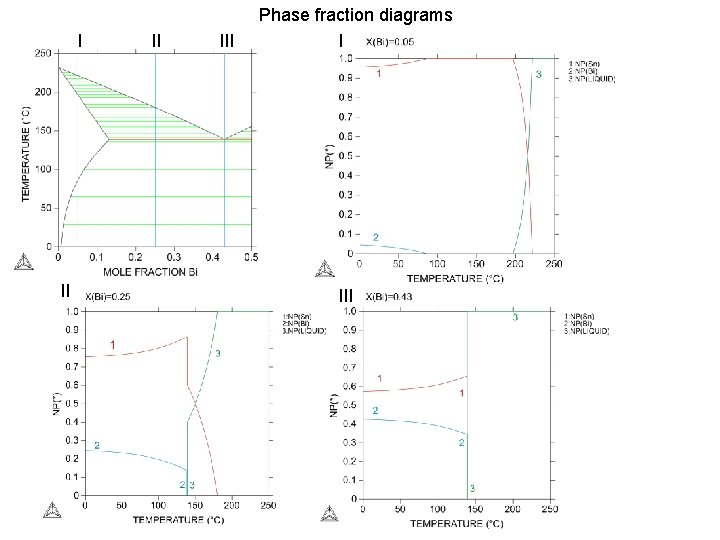
I II II III Phase fraction diagrams I III

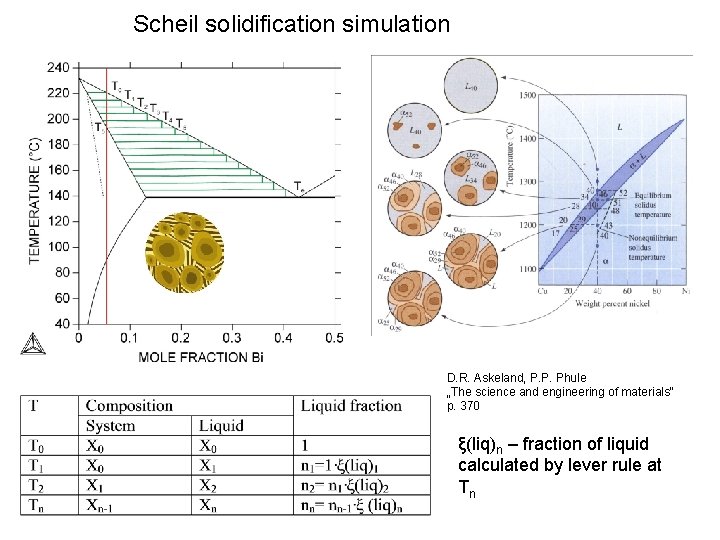
Scheil solidification simulation D. R. Askeland, P. P. Phule „The science and engineering of materials“ p. 370 ξ(liq)n – fraction of liquid calculated by lever rule at Tn

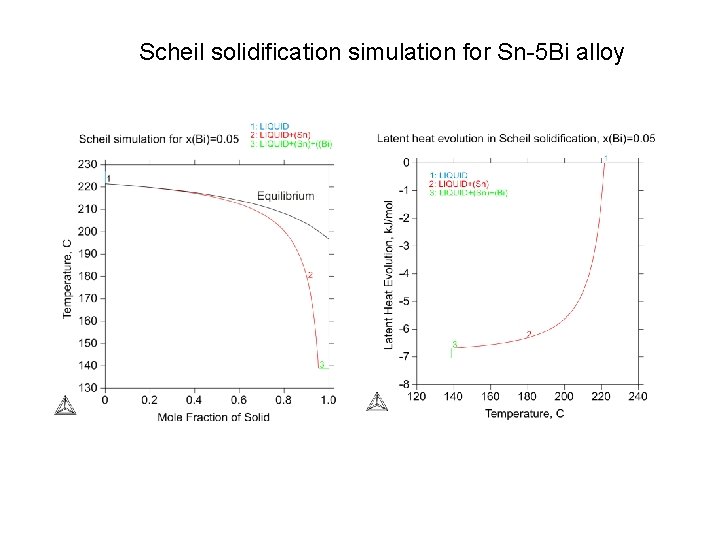
Scheil solidification simulation for Sn-5 Bi alloy

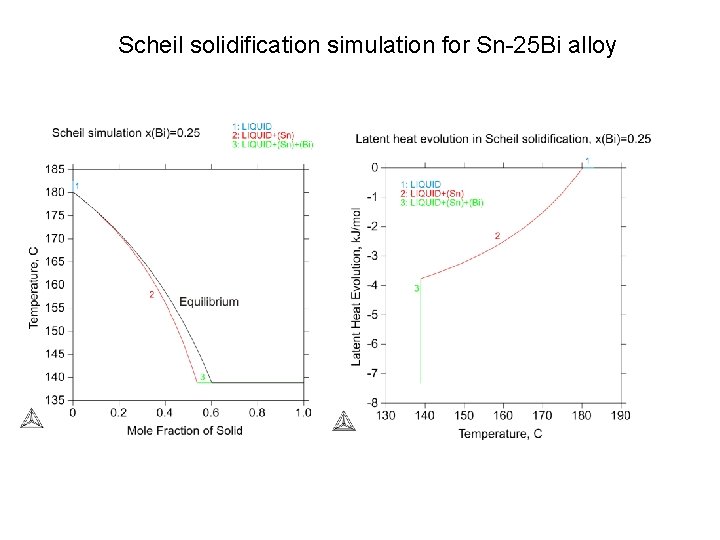
Scheil solidification simulation for Sn-25 Bi alloy

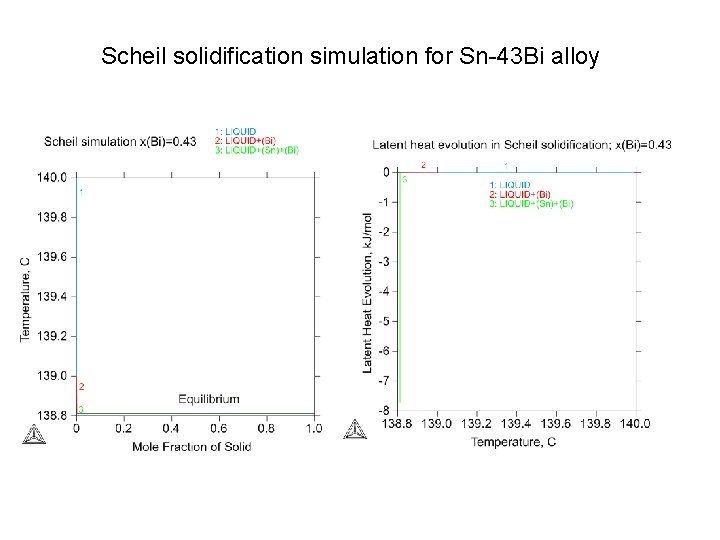
Scheil solidification simulation for Sn-43 Bi alloy

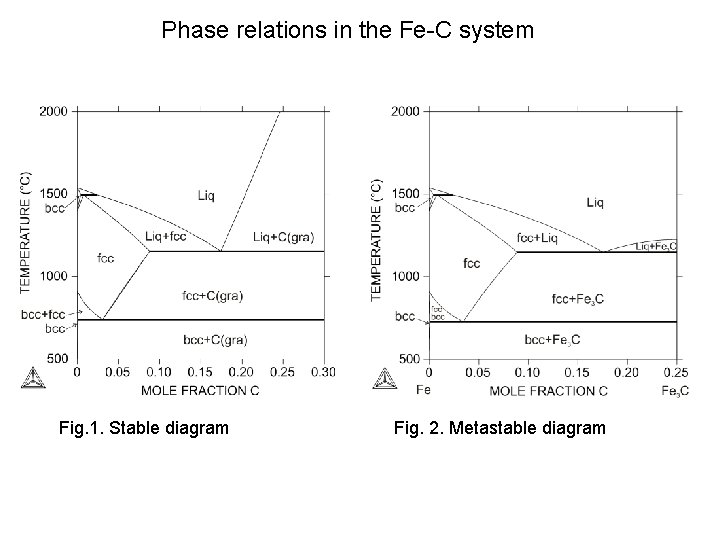
Phase relations in the Fe-C system Fig. 1. Stable diagram Fig. 2. Metastable diagram