Aqueous Solutions Lesson 1 Types of Solutions Electrolytes




- Slides: 9

Aqueous Solutions Lesson 1 Types of Solutions & Electrolytes and Nonelectrolytes YOUR TASKS Based on the content in the first two slides, take Quiz on Socrative: RENURAJAS 1 Based on the content in the last four slides, take Quiz on Socrative: RENURAJAS 2

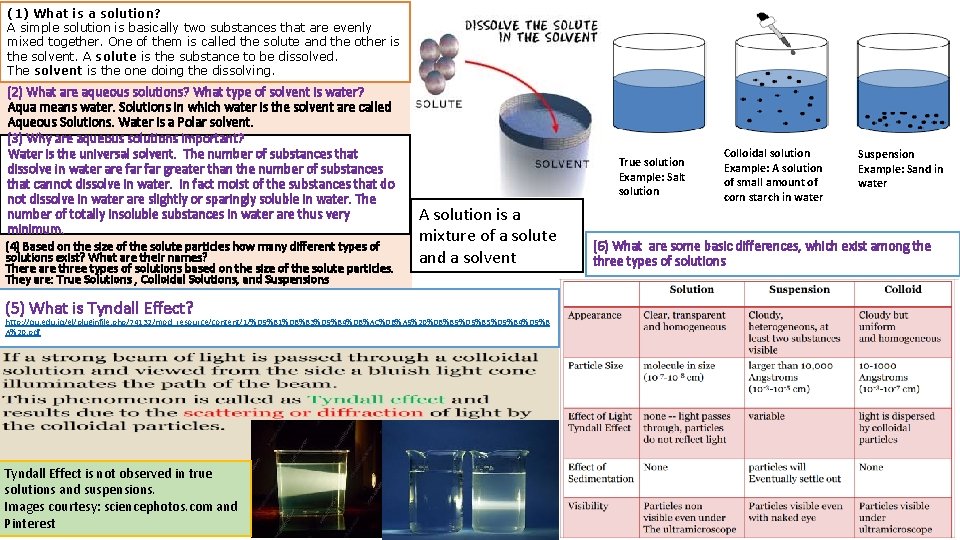
(1) What is a solution? A simple solution is basically two substances that are evenly mixed together. One of them is called the solute and the other is the solvent. A solute is the substance to be dissolved. The solvent is the one doing the dissolving. (2) What are aqueous solutions? What type of solvent is water? Aqua means water. Solutions in which water is the solvent are called Aqueous Solutions. Water is a Polar solvent. (3) Why are aqueous solutions important? Water is the universal solvent. The number of substances that dissolve in water are far greater than the number of substances that cannot dissolve in water. In fact moist of the substances that do not dissolve in water are slightly or sparingly soluble in water. The number of totally insoluble substances in water are thus very minimum. (4) Based on the size of the solute particles how many different types of solutions exist? What are their names? There are three types of solutions based on the size of the solute particles. They are: True Solutions , Colloidal Solutions, and Suspensions (5) What is Tyndall Effect? True solution Example: Salt solution A solution is a mixture of a solute and a solvent http: //qu. edu. iq/el/pluginfile. php/74132/mod_resource/content/1/%D 9%81%D 8%B 3%D 9%84%D 8%AC%D 8%A 9%20%D 8%B 9%D 9%85%D 9%84%D 9%8 A%20. pdf Tyndall Effect is not observed in true solutions and suspensions. Images courtesy: sciencephotos. com and Pinterest Colloidal solution Example: A solution of small amount of corn starch in water Suspension Example: Sand in water (6) What are some basic differences, which exist among the three types of solutions

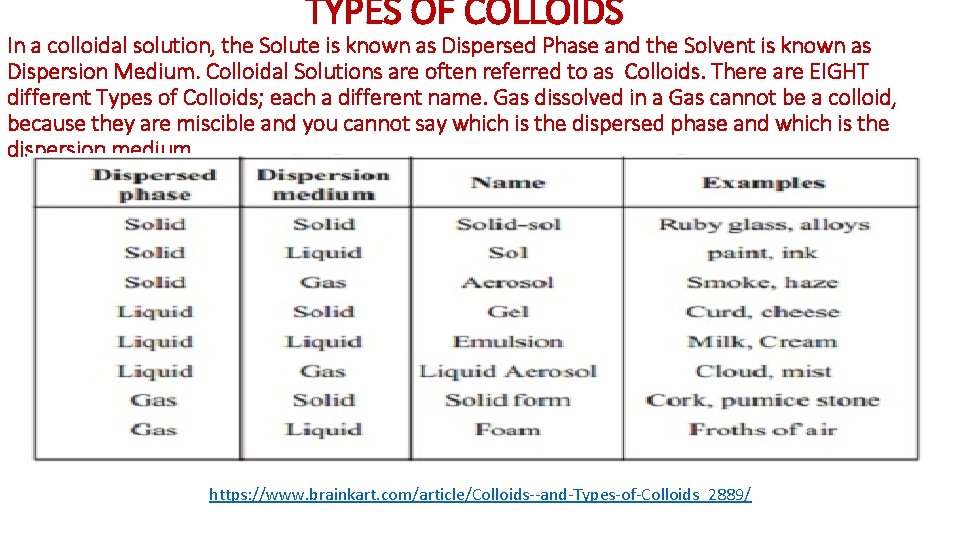
TYPES OF COLLOIDS In a colloidal solution, the Solute is known as Dispersed Phase and the Solvent is known as Dispersion Medium. Colloidal Solutions are often referred to as Colloids. There are EIGHT different Types of Colloids; each a different name. Gas dissolved in a Gas cannot be a colloid, because they are miscible and you cannot say which is the dispersed phase and which is the dispersion medium https: //www. brainkart. com/article/Colloids--and-Types-of-Colloids_2889/

YOUR TURN TAKE QUIZ ON SOCRATIVE: RENURAJAS 1

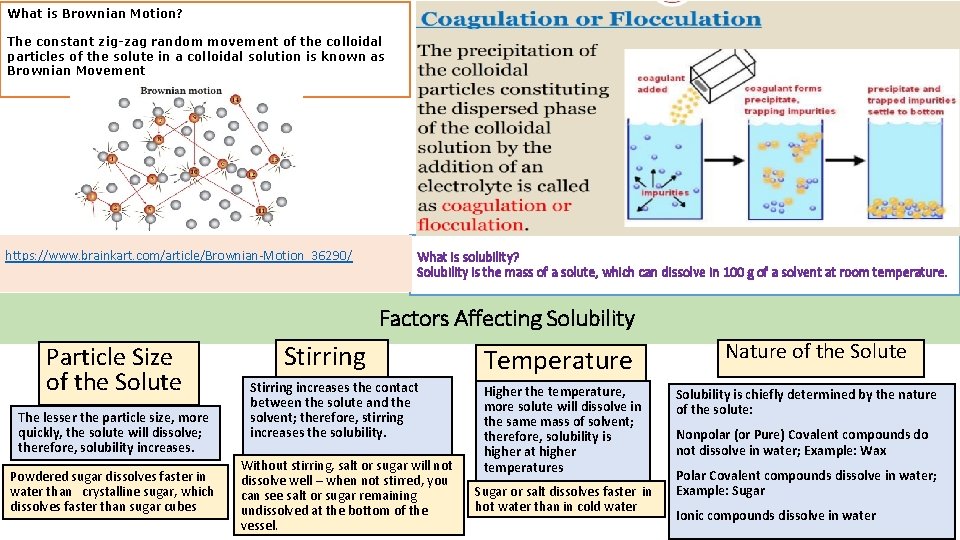
What is Brownian Motion? The constant zig-zag random movement of the colloidal particles of the solute in a colloidal solution is known as Brownian Movement https: //www. brainkart. com/article/Brownian-Motion_36290/ What is solubility? Solubility is the mass of a solute, which can dissolve in 100 g of a solvent at room temperature. Factors Affecting Solubility Particle Size of the Solute The lesser the particle size, more quickly, the solute will dissolve; therefore, solubility increases. Powdered sugar dissolves faster in water than crystalline sugar, which dissolves faster than sugar cubes Stirring increases the contact between the solute and the solvent; therefore, stirring increases the solubility. Without stirring, salt or sugar will not dissolve well – when not stirred, you can see salt or sugar remaining undissolved at the bottom of the vessel. Temperature Higher the temperature, more solute will dissolve in the same mass of solvent; therefore, solubility is higher at higher temperatures Sugar or salt dissolves faster in hot water than in cold water Nature of the Solute Solubility is chiefly determined by the nature of the solute: Nonpolar (or Pure) Covalent compounds do not dissolve in water; Example: Wax Polar Covalent compounds dissolve in water; Example: Sugar Ionic compounds dissolve in water

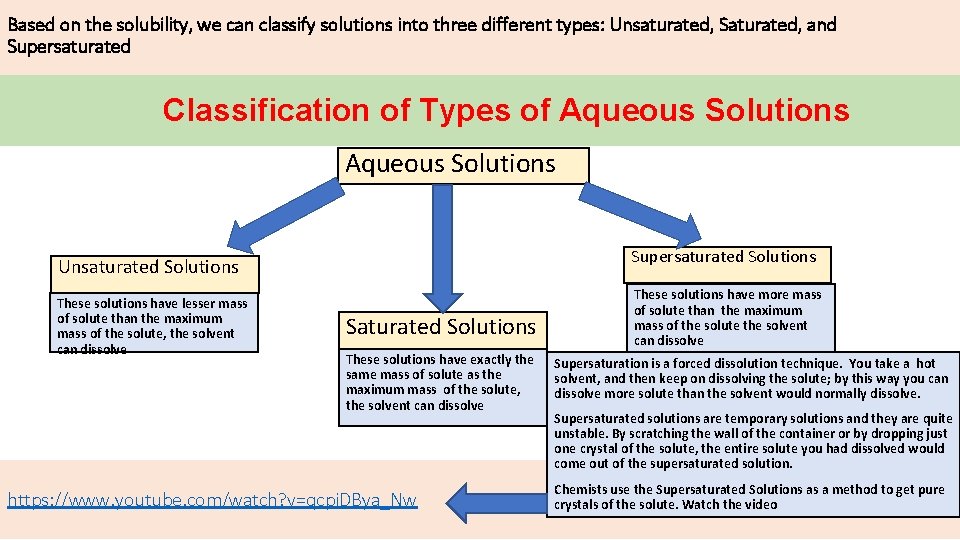
Based on the solubility, we can classify solutions into three different types: Unsaturated, Saturated, and Supersaturated Classification of Types of Aqueous Solutions Supersaturated Solutions Unsaturated Solutions These solutions have lesser mass of solute than the maximum mass of the solute, the solvent can dissolve Saturated Solutions These solutions have exactly the same mass of solute as the maximum mass of the solute, the solvent can dissolve https: //www. youtube. com/watch? v=qcpi. DBya_Nw These solutions have more mass of solute than the maximum mass of the solute the solvent can dissolve Supersaturation is a forced dissolution technique. You take a hot solvent, and then keep on dissolving the solute; by this way you can dissolve more solute than the solvent would normally dissolve. Supersaturated solutions are temporary solutions and they are quite unstable. By scratching the wall of the container or by dropping just one crystal of the solute, the entire solute you had dissolved would come out of the supersaturated solution. Chemists use the Supersaturated Solutions as a method to get pure crystals of the solute. Watch the video

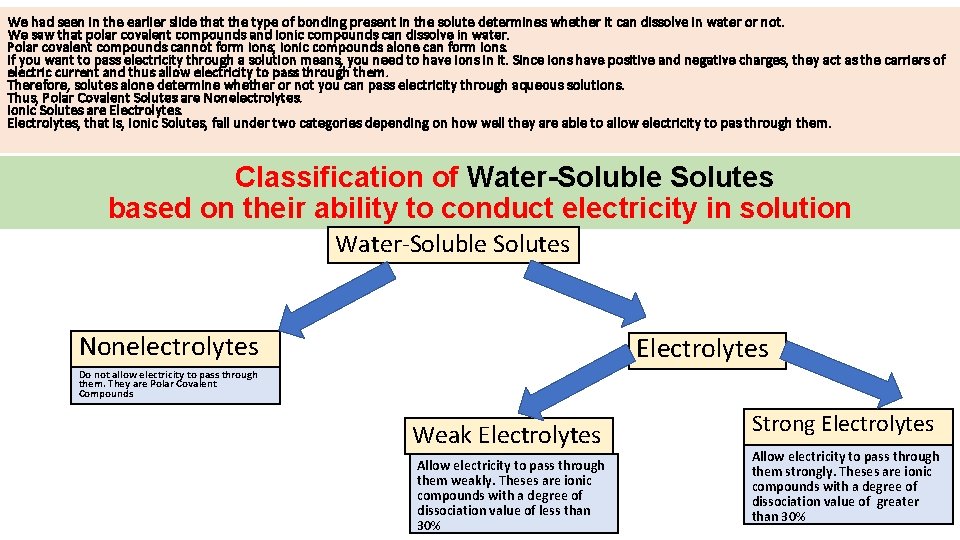
We had seen in the earlier slide that the type of bonding present in the solute determines whether it can dissolve in water or not. We saw that polar covalent compounds and ionic compounds can dissolve in water. Polar covalent compounds cannot form ions; ionic compounds alone can form ions. If you want to pass electricity through a solution means, you need to have ions in it. Since ions have positive and negative charges, they act as the carriers of electric current and thus allow electricity to pass through them. Therefore, solutes alone determine whether or not you can pass electricity through aqueous solutions. Thus, Polar Covalent Solutes are Nonelectrolytes. Ionic Solutes are Electrolytes, that is, Ionic Solutes, fall under two categories depending on how well they are able to allow electricity to pas through them. Classification of Water-Soluble Solutes based on their ability to conduct electricity in solution Water-Soluble Solutes Nonelectrolytes Electrolytes Do not allow electricity to pass through them. They are Polar Covalent Compounds Weak Electrolytes Allow electricity to pass through them weakly. Theses are ionic compounds with a degree of dissociation value of less than 30% Strong Electrolytes Allow electricity to pass through them strongly. Theses are ionic compounds with a degree of dissociation value of greater than 30%

EQUILIBRIUM IN ELECTROLYTE SOLUTIONS When electrolytes dissolve in water, there is an equilibrium between the ionic compound and its ions. Let us take an electrolyte AB, made up of the A+ cation and B- Anion. When such an electrolyte is dissolved in water, then the equilibrium between AB and A+ and B- will be represented by the following equilibrium: AB(aq) ⇌ A+ (aq) + B- (aq) This means that the ions can combine back to give the unsplit electrolyte. If the extent of equilibrium is 30% and above, then such an electrolyte is a strong electrolyte, If the extent of equilibrium is below 30% in the forward direction, then such an electrolyte is a weak electrolyte. The extent of the equilibrium is called the Degree of Dissociation. The value of 30% can also be given as 0. 30.

YOUR TURN TAKE QUIZ ON SOCRATIVE: RENURAJAS 2
 Reactions in aqueous solutions
Reactions in aqueous solutions Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Aqueous solutions module
Aqueous solutions module Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Ions in aqueous solutions and colligative properties
Ions in aqueous solutions and colligative properties General properties of aqueous solutions
General properties of aqueous solutions Calculate the number of grams of al in 371g of al2o3
Calculate the number of grams of al in 371g of al2o3 Electrical conductivity of aqueous solutions
Electrical conductivity of aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions