ANTICANCER AND ANTIHIV DRUGS DERIVED FROM AFRICAN AND






















- Slides: 27

ANTICANCER AND ANTI-HIV DRUGS DERIVED FROM AFRICAN AND OTHER PLANTS Gordon Cragg, Ph. D. NIH Special Volunteer Natural Products Branch Developmental Therapeutics Program Division of Cancer Treatment and Diagnosis NCI-Frederick Fairview Center, Suite 206 P. O. Box B Frederick, MD 21702 -1201, U. S. A. Phone: 301 -846 -5387; fax: 301 -846 -6178 e-mail: craggg@mail. nih. gov website: http: //dtp. nci. nih. gov; http: //dtp. nci. nih. gov/branches/npb/index. html

Traditional Medicine and Drug Discovery* • 80% of the world population resides in developing countries • 80% of people in developing countries utilize plants to meet their primary health care needs • Global pop. ca. 6. 3 billion – ca. 4 billion people utilize plants to meet their primary health care needs *Farnsworth NR, et al. Medicinal Plants in Therapy. Bull. W. H. O. 63: 965 -981 (1985)

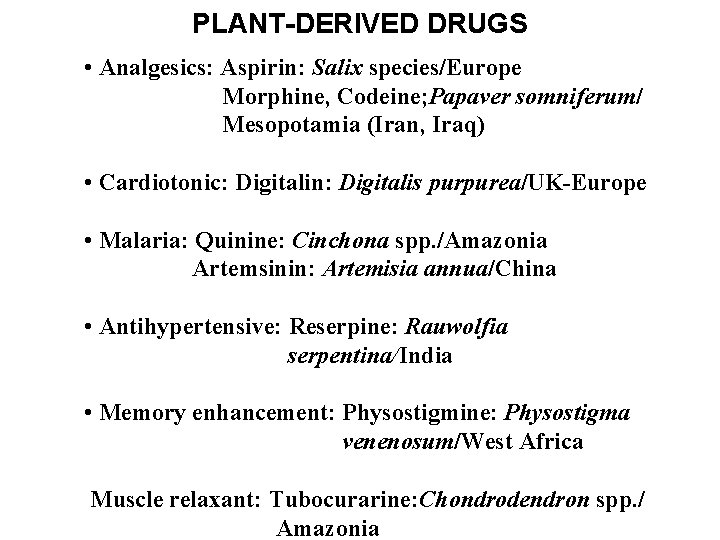
PLANT-DERIVED DRUGS • Analgesics: Aspirin: Salix species/Europe Morphine, Codeine; Papaver somniferum/ Mesopotamia (Iran, Iraq) • Cardiotonic: Digitalin: Digitalis purpurea/UK-Europe • Malaria: Quinine: Cinchona spp. /Amazonia Artemsinin: Artemisia annua/China • Antihypertensive: Reserpine: Rauwolfia serpentina/India • Memory enhancement: Physostigmine: Physostigma venenosum/West Africa Muscle relaxant: Tubocurarine: Chondrodendron spp. / Amazonia

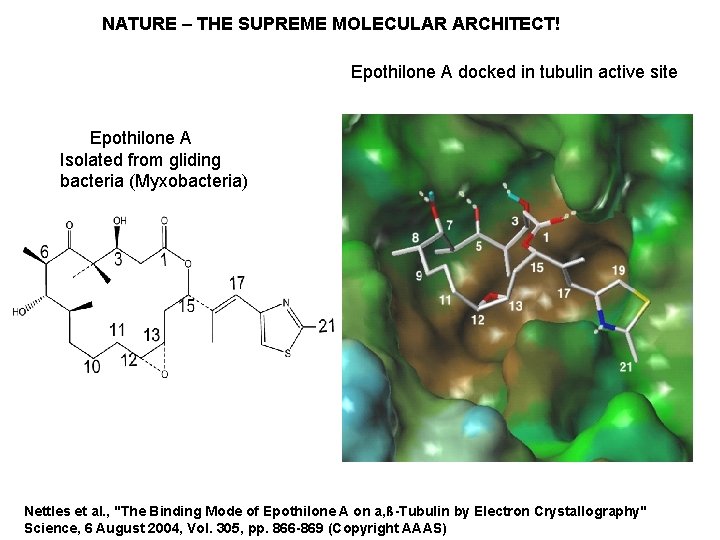
NATURE – THE SUPREME MOLECULAR ARCHITECT! Epothilone A docked in tubulin active site Epothilone A Isolated from gliding bacteria (Myxobacteria) Nettles et al. , "The Binding Mode of Epothilone A on a, ß-Tubulin by Electron Crystallography" Science, 6 August 2004, Vol. 305, pp. 866 -869 (Copyright AAAS)

PLANT-DERIVED ANTICANCER DRUGS IN CLINICAL USE OR DEVELOPMENT • Vinblastine/Vincristine: Catharanthus roseus/Jamaica, Philippines (originally from Madagascar) • Etoposide: Podophyllum species/ Eastern US, Himalayas • Paclitaxel/Docetaxel: Taxus species/NW US, Europe • Topotecan/Irinotecan: Camptotheca acuminata/China • Homoharringtonine: Cephalotaxus harringtonia/China • Flavopiridol: Synthetic based on rohutikine from Dysoxylum binectariferum/India • Combretastatins: Combretum caffrum/S. Africa

INTERNATIONAL COLLABORATION • Prior informed consent/permits from Source Country Government and stakeholders. • Collaboration with Source Country Organizations. • Training and technology transfer. • Protection of environment and sustainable development. • Plans for benefit-sharing Dr. D. Soejarto U. Illinois at Chicago

MICHELLAMINE B Potential Anti-AIDS Agent Discovery and Development • 1987: Collected liana Ancistrocladus korupensis leaves. Korup National Park, Mundemba, S. West Cameroon. Dr. Duncan Thomas (MBG) and Mr. Ndembe (Forestry Dept. ). • New species (Thomas & Gereau, Novon, 1993, 3, 494 -498). • 1989: Michellamine B isolated. Active against a range of HIV-1 and HIV-2 strains (Boyd et al. , J. Med. Chem, 1994, 37, 1740 -45). • Sufficient isolated from fallen leaves for preclinical development.


MICHELLAMINE B COLLABORATION: CAMEROON

FEASIBILITY STUDY CULTIVATION OF A. KORUPENSIS

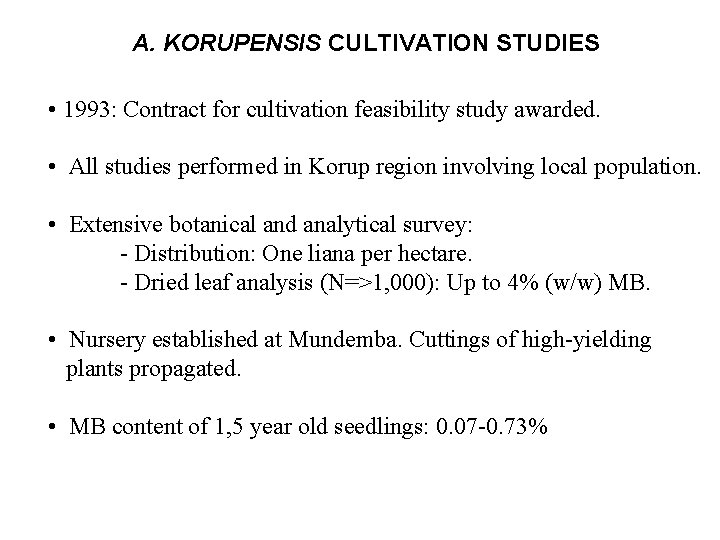
A. KORUPENSIS CULTIVATION STUDIES • 1993: Contract for cultivation feasibility study awarded. • All studies performed in Korup region involving local population. • Extensive botanical and analytical survey: - Distribution: One liana per hectare. - Dried leaf analysis (N=>1, 000): Up to 4% (w/w) MB. • Nursery established at Mundemba. Cuttings of high-yielding plants propagated. • MB content of 1, 5 year old seedlings: 0. 07 -0. 73%


DEVELOPMENT OF MICHELLAMINE B • Formulation as diacetate salt. • Toxicology: Rodents, dogs, primates. Toxic dose level close to anticipated effective antiviral dose (narrow therapeutic index). • Development suspended. • Possibility of lead development (Bringmann, Wurzburg). • Novel antimalarial agents, the korupensamines, add further promise for A. korupensis.

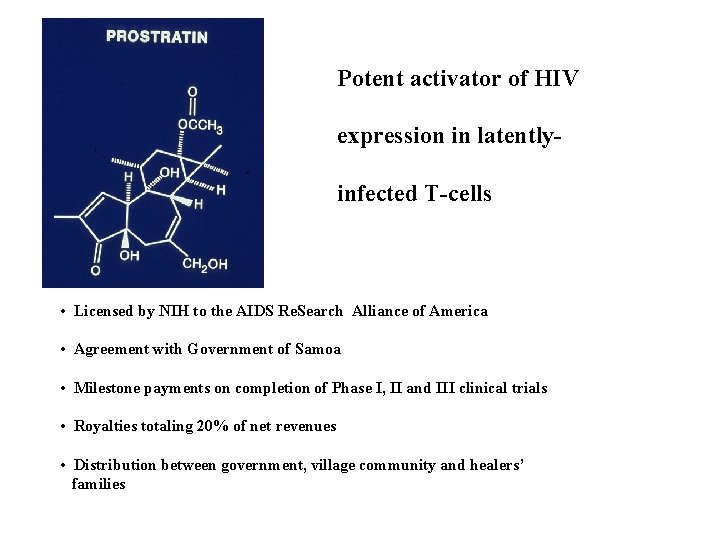
POTENTIAL ANTI-HIV DRUG FROM HOMALANTHUS NUTANS : PROSTRATIN • Dr. Paul Cox entered into a Covenant with healers of Western Samoan village of Falealupo • Covenant signed with village chiefs and orators with concurrence of Prime Minister and Parliament. • Use of Homalanthus nutans by healers recorded by Dr. Cox: Treatment of “yellow” fever. • $480, 000 provided for schools, clinics, water supplies, trails, aerial walkways, etc. • Endowment established for preservation of forest. P. A. Cox, Pharmaceutical Biology, 2001, 39 (Supplement ), 33 -40

Samoan Traditional Healers in Village of Falealupo

Potent activator of HIV expression in latentlyinfected T-cells • Licensed by NIH to the AIDS Re. Search Alliance of America • Agreement with Government of Samoa • Milestone payments on completion of Phase I, II and III clinical trials • Royalties totaling 20% of net revenues • Distribution between government, village community and healers’ families

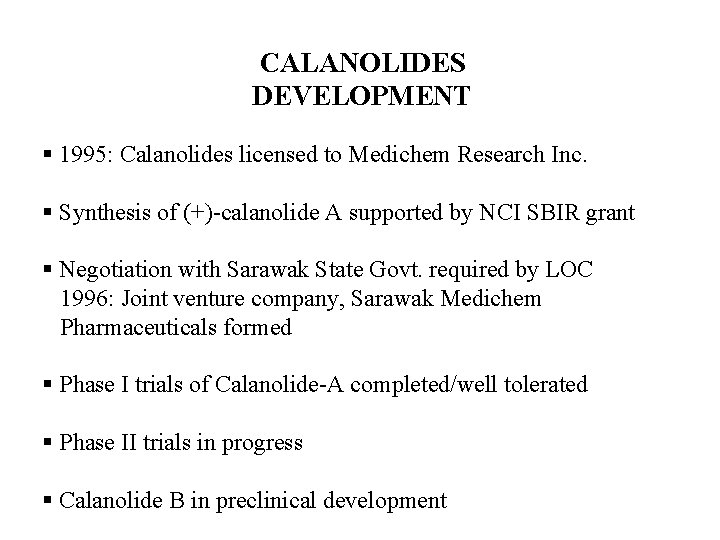
Calophyllum teysmannii var. inophylloide. Sustainable source of potential anti-AIDS drug, calanolide B. Discovery from tree in Sarawak, Malaysia, promoted conservation and replanting of seedlings in clearcut regions, and led to establishment of the Sarawak Biodiversity Center for in-country research on drug discovery from local biodiversity D. D. Soejarto, University of Illinois at Chicago

CALANOLIDES DEVELOPMENT § 1995: Calanolides licensed to Medichem Research Inc. § Synthesis of (+)-calanolide A supported by NCI SBIR grant § Negotiation with Sarawak State Govt. required by LOC 1996: Joint venture company, Sarawak Medichem Pharmaceuticals formed § Phase I trials of Calanolide-A completed/well tolerated § Phase II trials in progress § Calanolide B in preclinical development

PARALLEL DEVELOPMENT OF HERBAL MEDICINES AND THE DISCOVERY OF NOVEL CONVENTIONAL DRUGS Bioassay-guided isolation and chemical characterization of active principle(s) • Provide markers for standardization of herbal products • Provide lead compounds for conventional drug development

Basic Philosophy Any herbal drug or botanical supplement to be considered for clinical trials must be botanically authenticated as well as chemically and biologically standardized. Dr. Norman Farnsworth, Director, UIC/NIH Center for Botanical Dietary Supplements Research

Steps Required Prior to Clinical Assessment of Herbal Drugs/Botanical Dietary Supplements 1. Acquire plant material • Verify identity; taxonomic/microscopic/PCR • Check for pesticides; herbicides; heavy metals 2. Establish/select appropriate bioassay 3. Bioassay several types of extracts • In vitro • In vivo (if possible/relevant)

Steps Required (continued) 4. Bioassay-guided isolation and chemical characterization of active principle(s) 5. Prepare the biologically and chemically standardized dosage form • 6. Conduct stability studies In vitro studies on standardized product • Metabolism (including interactions with p 450 s) • Pharmacokinetics • Toxicity • Mechanism of Action

http: //www. asnapp. org/ Thank you for visiting this website, which is intended to be a network hub for all stakeholders in Africa’s natural plant products sector. Through knowledge-sharing and information-exchange via this site, ASNAPP (Agribusiness in Sustainable Natural African Plant Products) seeks to create a knowledge community that will strengthen the continent’s capacity to develop this sector. In the interests of developing successful natural product agribusinesses, and thus helping to reduce poverty in rural communities, ASNAPP promotes collaboration and knowledge sharing with research and academic institutions, government, private enterprises, non-profit organizations, the donor community and civil Society. For this purpose, the ASNAPP website encourages information exchange in any of the following areas: Applied research and technology transfer Quality assurance and control Market linkages and development Enterprise and farmer association development Natural resource management Policy dialogue and advocacy

These areas are the main thrust of ASNAPP’s activities for 2005, based on the following three new programmes funded by the United States Agency for International Development (USAID): The Partnership for Food Industry Development Program (PFID), managed by Rutgers University’s New Use Agriculture and Natural Plants Products Program. The Rural Livelihoods Activity in Southern Africa programme, run by the Michigan State University Partnership for Food Industry Develop Program – Fruits and Vegetables (MSU PFID – F & V) in conjunction with a consortium of partners. The Partnership for Sustainable Germplasm Development for Non-traditional Crops, a collaborative project involving various academic and research institutions as well as private enterprises in South Africa and Zambia. ASNAPP USARutgers University - Cook College Department of Plant Biology and Plant Pathology 59 Dudley Road, 381 Foran Hall New Brunswick, New Jersey 08901 Professor Jim Simon Co-Principal Investigator and Quality Control Coordinator ASNAPP Program New Use Agriculture and Natural Plants Program Tel: +732 932 -9711 Ext. 355/379 Fax: +732 932 -9377 Email: jesimon@aesop. rutgers. edu Website: www. nuanpp. org Partner countries: Ghana, Rwanda, Senegal, South Africa, Zambia, USA

Clinical Trials and Complementary and Alternative Medicine (CAM) While many CAM treatments have already been in use for a long time (sometimes for centuries), there is not the kind of scientific knowledge available about them that has been gained from studies of conventional medicine. Many people are already using CAM, and without this scientific knowledge, they may be at risk— for example, for serious effects from taking the wrong dose, using the treatment in the wrong way, or using it with another treatment with which it interacts.

INTERNATIONAL AND REGIONAL COLLABORATION AFASSA: Africa, Asia and South America • Co-ordinates activities of networks involved in natural product research in Africa, Asia and South America. • Founded at Intercontinental Symposium on Natural Products Research in Montevideo in December, 1999. NAPRECA SYMPOSIUM ADDIS ABABA 2003 Next symposium: Antananarivo, Madagascar August 9 -12, 2005 Takelaka. dts. mg/rafita

THANK YOU http: //dtp. nci. nih. gov
 Anticancer drugs classification
Anticancer drugs classification Anticancer drugs classification
Anticancer drugs classification Anthracyclines
Anthracyclines Pan african and independence comprehension check answers
Pan african and independence comprehension check answers Lesson 5 african american culture and politics
Lesson 5 african american culture and politics Center for african peace and conflict resolution
Center for african peace and conflict resolution An african thunderstorm worksheet
An african thunderstorm worksheet African mythology gods and goddesses
African mythology gods and goddesses North and central african societies
North and central african societies What are the elements of folktale?
What are the elements of folktale? African masks color meanings
African masks color meanings Person and community in african traditional thought
Person and community in african traditional thought Difference between african elephant and asian elephant
Difference between african elephant and asian elephant African charter on democracy, elections and governance
African charter on democracy, elections and governance West african society and culture section 3
West african society and culture section 3 African institute for economic development and planning
African institute for economic development and planning North and central african societies
North and central african societies Bloody life in my bloody hands
Bloody life in my bloody hands Base and derived quantities
Base and derived quantities Position fundamental
Position fundamental Slidetodoc.com
Slidetodoc.com Basic engineering definition
Basic engineering definition Difference between fundamental and derived unit
Difference between fundamental and derived unit Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Fundamental and derived positions
Fundamental and derived positions Acuity charting forms
Acuity charting forms Lliver
Lliver