AN INTRODUCTION TO FAJANS RULES A guide for











- Slides: 19

AN INTRODUCTION TO FAJAN’S RULES A guide for A level students KNOCKHARDY PUBLISHING 2008 SPECIFICATIONS

KNOCKHARDY PUBLISHING FAJAN’S RULES INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A 2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available. Accompanying notes on this, and the full range of AS and A 2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at. . . www. knockhardy. org. uk/sci. htm Navigation is achieved by. . . either or clicking on the grey arrows at the foot of each page using the left and right arrow keys on the keyboard

INTRODUCTION Observations Not all ionic compounds have high melting points. Some covalently bonded compounds have higher than expected boiling points due to dipoles in their structure Reason in many substances bonding is not 100% ionic or covalent

INTRODUCTION Observations Not all ionic compounds have high melting points. Some covalently bonded compounds have higher than expected boiling points due to dipoles in their structure Reason Ideal ionic compound in many substances bonding is not 100% ionic or covalent completely separate, spherical ions electron densities are apart from each other However, if the positive ion has a high charge density it can distort the negative ion by attracting the outer shell electrons to give an area of electron density between the two species. . . a bit like a covalent bond

INTRODUCTION The feasibility of having some covalent character can be predicted using Fajan’s Rules. A compound is more likely to be covalent if the. . . CATION SMALL SIZE HIGH CHARGE it is “highly polarising” and attracts electrons in the anion ANION LARGE SIZE HIGH CHARGE it is “highly polarisable” and will be easily distorted

INTRODUCTION The feasibility of having some covalent character can be predicted using Fajan’s Rules. A compound is more likely to be covalent if the. . . CATION SMALL SIZE HIGH CHARGE it is “highly polarising” and attracts electrons in the anion ANION LARGE SIZE HIGH CHARGE it is “highly polarisable” and will be easily distorted N. B. Just because a substance is less likely to be covalent according to Fajan’s Rules doesn’t mean it will be ionic; it will remain covalent but have some ionic character (or vice versa).

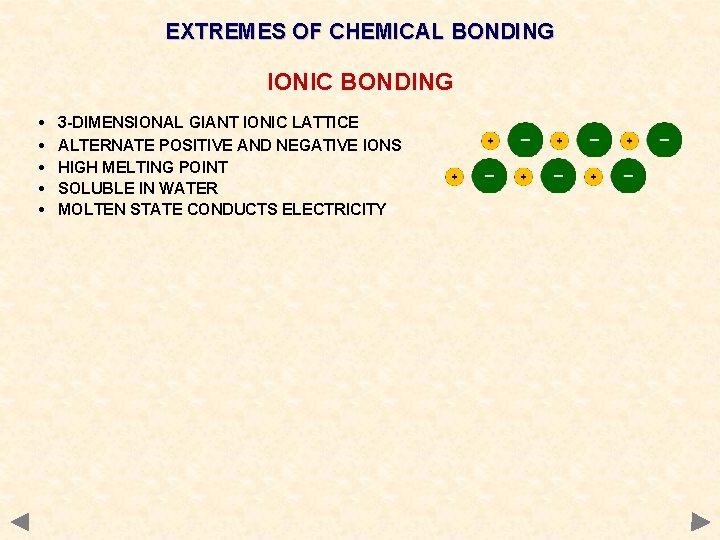
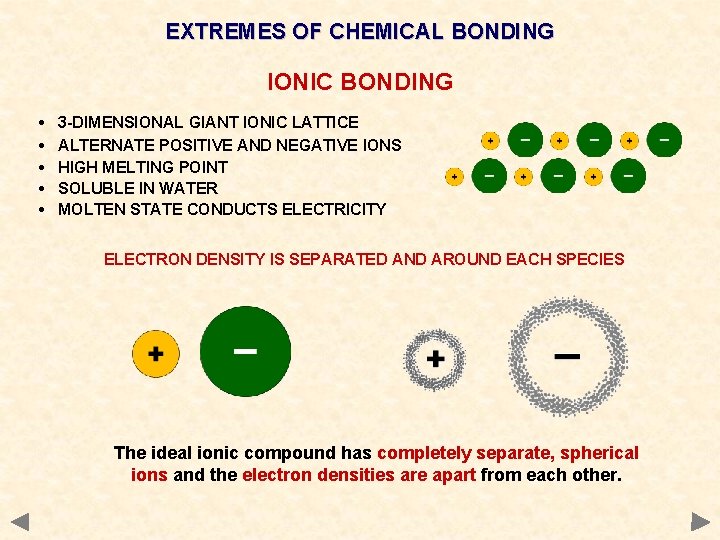
EXTREMES OF CHEMICAL BONDING IONIC BONDING • • • 3 -DIMENSIONAL GIANT IONIC LATTICE ALTERNATE POSITIVE AND NEGATIVE IONS HIGH MELTING POINT SOLUBLE IN WATER MOLTEN STATE CONDUCTS ELECTRICITY

EXTREMES OF CHEMICAL BONDING IONIC BONDING • • • 3 -DIMENSIONAL GIANT IONIC LATTICE ALTERNATE POSITIVE AND NEGATIVE IONS HIGH MELTING POINT SOLUBLE IN WATER MOLTEN STATE CONDUCTS ELECTRICITY ELECTRON DENSITY IS SEPARATED AND AROUND EACH SPECIES The ideal ionic compound has completely separate, spherical ions and the electron densities are apart from each other.

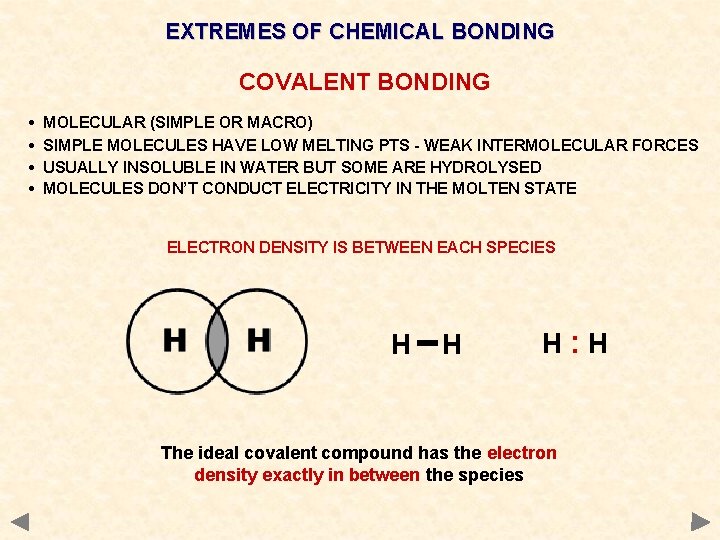
EXTREMES OF CHEMICAL BONDING COVALENT BONDING • • MOLECULAR (SIMPLE OR MACRO) SIMPLE MOLECULES HAVE LOW MELTING PTS - WEAK INTERMOLECULAR FORCES USUALLY INSOLUBLE IN WATER BUT SOME ARE HYDROLYSED MOLECULES DON’T CONDUCT ELECTRICITY IN THE MOLTEN STATE

EXTREMES OF CHEMICAL BONDING COVALENT BONDING • • MOLECULAR (SIMPLE OR MACRO) SIMPLE MOLECULES HAVE LOW MELTING PTS - WEAK INTERMOLECULAR FORCES USUALLY INSOLUBLE IN WATER BUT SOME ARE HYDROLYSED MOLECULES DON’T CONDUCT ELECTRICITY IN THE MOLTEN STATE ELECTRON DENSITY IS BETWEEN EACH SPECIES H H H: H The ideal covalent compound has the electron density exactly in between the species

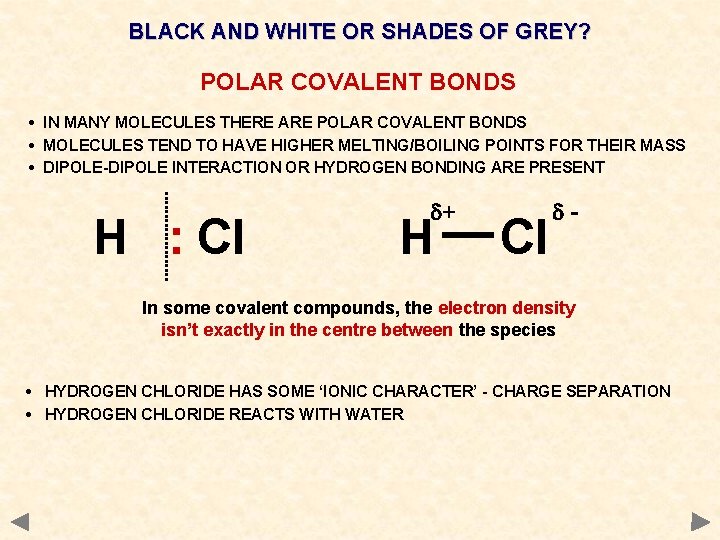
BLACK AND WHITE OR SHADES OF GREY? POLAR COVALENT BONDS • IN MANY MOLECULES THERE ARE POLAR COVALENT BONDS • MOLECULES TEND TO HAVE HIGHER MELTING/BOILING POINTS FOR THEIR MASS • DIPOLE-DIPOLE INTERACTION OR HYDROGEN BONDING ARE PRESENT H : Cl d+ H Cl d- In some covalent compounds, the electron density isn’t exactly in the centre between the species • HYDROGEN CHLORIDE HAS SOME ‘IONIC CHARACTER’ - CHARGE SEPARATION • HYDROGEN CHLORIDE REACTS WITH WATER

BLACK AND WHITE OR SHADES OF GREY? IONIC COMPOUNDS WHICH ‘MISBEHAVE’ • LITHIUM CHLORIDE SHOULD BEHAVE LIKE A TYPICAL GROUP I CHLORIDE • BUT… IT IS HYDROLYSED BY WATER AND HAS A ‘LOW’ MELTING POINT

BLACK AND WHITE OR SHADES OF GREY? IONIC COMPOUNDS WHICH ‘MISBEHAVE’ • LITHIUM CHLORIDE SHOULD BEHAVE LIKE A TYPICAL GROUP I CHLORIDE • BUT… IT IS HYDROLYSED BY WATER AND HAS A ‘LOW’ MELTING POINT THE POSITIVE ION ATTRACTS THE OUTER SHELL ELECTRONS OF THE NEGATIVE ION AND DISTORTS THE SPHERICAL IONIC SHAPE. . . THERE IS NOW SOME ELECTRON DENSITY BETWEEN THE SPECIES

BLACK AND WHITE OR SHADES OF GREY? IONIC COMPOUNDS WHICH ‘MISBEHAVE’ • LITHIUM CHLORIDE SHOULD BEHAVE LIKE A TYPICAL GROUP I CHLORIDE • BUT… IT IS HYDROLYSED BY WATER AND HAS A ‘LOW’ MELTING POINT THE POSITIVE ION ATTRACTS THE OUTER SHELL ELECTRONS OF THE NEGATIVE ION AND DISTORTS THE SPHERICAL IONIC SHAPE. . . THERE IS NOW SOME ELECTRON DENSITY BETWEEN THE SPECIES SMALL CATION LARGE ANION HIGH CHARGE DENSITY ELECTRONS FAR FROM NUCLEUS HIGHLY POLARISING HIGHLY POLARISABLE

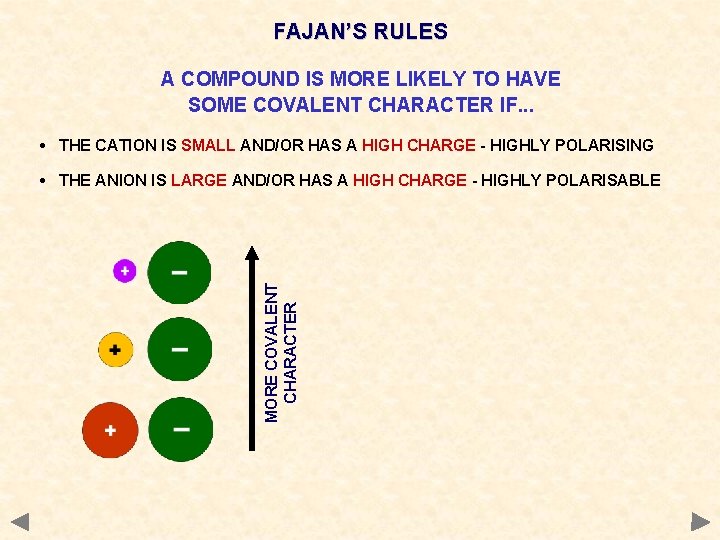
FAJAN’S RULES A COMPOUND IS MORE LIKELY TO HAVE SOME COVALENT CHARACTER IF. . . • THE CATION IS SMALL AND/OR HAS A HIGH CHARGE - HIGHLY POLARISING • THE ANION IS LARGE AND/OR HAS A HIGH CHARGE - HIGHLY POLARISABLE

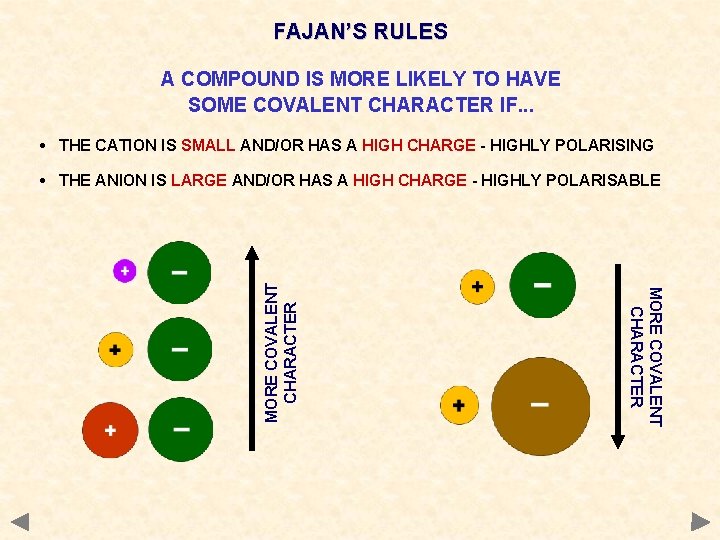
FAJAN’S RULES A COMPOUND IS MORE LIKELY TO HAVE SOME COVALENT CHARACTER IF. . . • THE CATION IS SMALL AND/OR HAS A HIGH CHARGE - HIGHLY POLARISING MORE COVALENT CHARACTER • THE ANION IS LARGE AND/OR HAS A HIGH CHARGE - HIGHLY POLARISABLE

FAJAN’S RULES A COMPOUND IS MORE LIKELY TO HAVE SOME COVALENT CHARACTER IF. . . • THE CATION IS SMALL AND/OR HAS A HIGH CHARGE - HIGHLY POLARISING MORE COVALENT CHARACTER • THE ANION IS LARGE AND/OR HAS A HIGH CHARGE - HIGHLY POLARISABLE

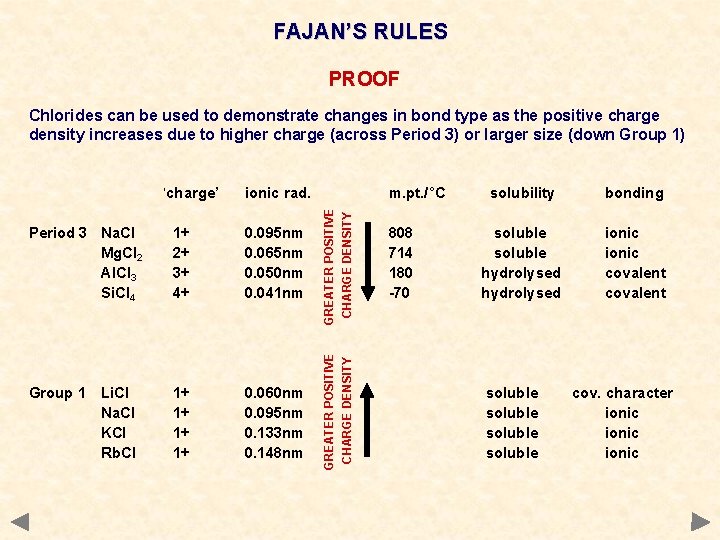
FAJAN’S RULES PROOF Chlorides can be used to demonstrate changes in bond type as the positive charge density increases due to higher charge (across Period 3) or larger size (down Group 1) Period 3 Na. Cl Mg. Cl 2 Al. Cl 3 Si. Cl 4 1+ 2+ 3+ 4+ 0. 095 nm 0. 065 nm 0. 050 nm 0. 041 nm Group 1 Li. Cl Na. Cl KCl Rb. Cl 1+ 1+ 0. 060 nm 0. 095 nm 0. 133 nm 0. 148 nm m. pt. /°C GREATER POSITIVE CHARGE DENSITY ionic rad. GREATER POSITIVE CHARGE DENSITY ‘charge’ 808 714 180 -70 solubility bonding soluble hydrolysed ionic covalent soluble cov. character ionic

AN INTRODUCTION TO FAJAN’S RULES THE END © 2008 JONATHAN HOPTON & KNOCKHARDY PUBLISHING